Abstract
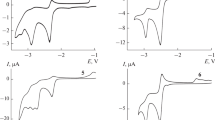
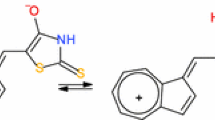
The electrochemical behavior of substituted 1-phenylselanyl azulenes has been established by cyclic, differential pulse, and rotating disk electrode voltammetric methods. The redox potentials and the number of electrons exchanged in the main redox processes have been examined in connection with the electronic effects of their substituents. Controlled potential electrolyses have been performed in order to point out the products associated to the redox peaks. A mechanism to explain the redox processes has been proposed.












Similar content being viewed by others
References
Jaworski JS (2012) Electrochemistry of organic selenium and tellurium compounds. Patai’s Chemistry of Functional Groups John Wiley & Sons.
Jin J, Li B, Lorance E, Okumura N, Macia-Ruvalcaba N, Zakai U, Zhang S-Z, Block E, Glass R (2010) Electrochemical and chemical oxidation of dithia-, diselena-, ditellura-, selenathia-, and tellurathiamesocycles and stability of the oxidized species. J Org Chem 75:1997–2009
Detty MR, Logan ME (2004) One- and two-electron oxidations and reductions of organoselenium and organotellurium compounds. Adv Phys Or g Chem 39:79–145
Degrand C, Gautier C, Prest R (1988) Cathodic reduction of diphenyl selenide in acetonitmle. J Electroanal Chem 248:381–386
Tepecik A, Altin Z, Erturan S (2008) The diorganoselenium and selenides compounds electrochemistry. J Autom Methods Manag Chem, Article ID 839153:6
Combs G, Levander O, Spallholtz J, Oldfield J (1987) Selenium in biology and medicine. AVI, Westport, CT
Lu J, Jiang C, Kaeck M, Ganther H, Ip C, Thompson H (1995) Cellular and metabolic effects of triphenylselenonium chloride in a mammary cell culture model. Carcinogenesis 16:513–517
Soriano-Garcia M (2004) Organoselenium compounds as potential therapeutic and chemopreventive agents. A Review Curr Med Chem 11:1657–1669
Mlochowski J, Kloc K, Lisiak R, Potaczek P, Wojtowicz H (2007) Developments in the chemistry of selenaheterocyclic compounds of practical importance in synthesis and medicinal biology. Arkivoc vi:14–46
Pietka-Ottlik M, Wojtowicz-Mlochowska H, Kolodziejczyk K, Piasecki E, Mlochowski J (2008) New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem Pharm Bull 56:1423–1427
Ip C, Lisk DJ, Ganther H, Thompson H (1997) Triphenylselenonium and diphenylselenide in cancer chemoprevention: comparative studies of anticarcinogenic efficacy, tissue selenium levels and excretion profile. J Anticancer Res 17:3195–3199
Nefedov VA (1968) Substitution reaction with copper salts. IV. Formation of C-Se and C-Te bonds. J Gen Chem [USSR] 38:2122–2123
Edson d A, dos S, Ernest H, Ruoli B, James CB, Camila S, Suniga T, Danielle B, Renata TP, Alexandra M, Antunes M, Matilde M, Marques M, de F, Matos C, Dênis P, de L (2013) Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorg Med Chem Lett 23:4669–4673
Razus AC, Birzan L, Hanganu A, Cristea M, Enache C, Ungureanu E-M, Soare ML (2014) 1-Phenylselanylazulenes: synthesis and selenium atom oxidation. Monatsh Chem 145:1999–2009
Razus AC, Birzan L, Cristea M, Dragu EA, Hanganu A (2011) Azulen-1-yl diazenes substituted at C-3 with phenyl-chalcogene moieties: dye synthesis, product characterization and properties. Monatsh Chem 142:1271–1282
Inel GA, Soare ML, Bujduveanu MR, Varga Ş, Ungureanu EM, Birzan L (2014) Electrochemical characterization of some azulene selenium compounds. U.P.B. Sci Bull Series B 76:3–10
Inel GA, Cioates NC, Mirsky V, Birzan L, Ungureanu EM (2015) Films obtained by electrochemistry from (6-methylazulene-1-yl) (phenyl) selane. U.P.B. Sci Bull Series B 77:3–18
Buica GO, Ungureanu EM, Birzan L, Razus AC, Bujduveanu MR (2011) Films of poly (4-azulen-1-yl-2,6-bis (2-thienyl) pyridine) for heavy metal ions complexation. Electrochim Acta 56:5028–5036
Jouikov VV, Fattakhova DS, Kozhevnikov AY (2001) The potential-determining reaction of electrogenerated cation radicals of diphenylselenide: dimerization versus disproportionation. Electrochim Acta 46:807–812
Engman L, Perssor J, Andersson CM, Berglund M (1992) Application of the Hammett equation to the electrochemical oxidation of diaryl chalcogenides and aryl methyl chalcogenides. J Chem Soc Perkin Trans 2:1309–1313
Engman L, Lind J, Merenyi G (1994) Redox properties of diaryl chalcogenides and their oxides. J Phys Chem 98:3174–3182
Jouikov V (1995) Anodic reactivity of alkylphenylselenides. J Electroanal Chem 398:159–164
Shoji T, Okada K, Ito S, Toyota K, Morita N (2010) Synthesis of 1-(pyridyl, quinolyl, and isoquinolyl) azulenes by Reissert–Henze type reaction. Tetrahedron Lett 51:5127–5130
Shoji T, Ito S, Toyota K, Yasunami M, Morita N (2010) The novel transition metal free synthesis of 1,1′-biazulene. Tetrahedron Lett 48:4999–5002
Shoji T, Higashi H, Ito S, Toyota K, Yasunami M, Morita N (2008) Synthesis and redox behavior of 1-azulenyl sulfides and efficient synthesis of 1,1′-biazulenes. Eur J Org Chem 7:1242–1252
Shoji T, Ito S, Toyota K, Iwamoto T, Yasunami M, Morita N (2009) Synthesis and redox behavior of 1,3-bismethylthio-) and 1,3-bis (phenylthio) azulenes bearing 2- and 3-thienyl substituents by palladium-catalyzed cross-coupling reaction of 2- and 6-haloazulenes with thienylmagnesium ate complexes. Eur J Org Chem 25:4307–4315
Acknowledgments
The authors are grateful for the financial support from the UEFISCDI projects PN-II-ID-PCE-2011-3-1067 Ctr. No. 15/2011, PN-II-PT-PCCA-2013-4-2151 Ctr. No. 236/2014, and ID PN-II-RU-TE-2014-4-0594 Ctr. No. 10/2015, and the work done by Drd. Georgiana Anca Inel has been supported by the Sectorial Operational Programme Human Resources Development 2007–2013 (InnoRESEARCH) 132395, financed by the European Social Fund and the Romanian Government under the contract number POSDRU/159/1.5/S/132395.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Fig S1
(DOCX 149 kb)
Fig S2
(DOCX 182 kb)
Fig S3
(DOCX 119 kb)
Fig S4
(DOCX 156 kb)
Fig S5
(DOCX 188 kb)
Fig S6
(DOCX 170 kb)
Fig S7
(DOCX 189 kb)
Fig S8
(DOCX 203 kb)
Fig S9
(DOCX 193 kb)
Fig S10
(DOCX 169 kb)
Fig S11
(DOCX 86 kb)
Fig S12
(DOCX 86 kb)
Fig S13
(DOCX 370 kb)
Fig S14
(DOCX 56 kb)
Fig S15
(DOCX 90 kb)
Fig S16
(DOCX 76 kb)
Fig S17
(DOCX 83 kb)
Fig S18
(DOCX 71 kb)
Fig S19
(DOCX 72 kb)
Fig S20
(DOCX 82 kb)
Fig S21
(DOCX 91 kb)
Fig S22
(DOCX 107 kb)
Fig S23
(DOCX 110 kb)
Fig S24
(DOCX 98 kb)
Fig S25
(DOCX 105 kb)
Fig S26
(DOCX 79 kb)
Fig S27
(DOCX 79 kb)
Rights and permissions
About this article
Cite this article
Buica, GO., Soare, ML., Inel, G.A. et al. On the electrochemical behavior of selanyl azulenes. J Solid State Electrochem 20, 3151–3164 (2016). https://doi.org/10.1007/s10008-016-3371-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3371-8