Abstract
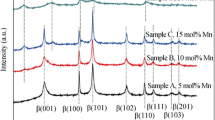
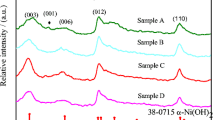
β-Nickel hydroxide was successfully synthesized by a hydrothermal method. Nano-nickel hydroxide material was characterized by X-ray diffraction, infrared absorption spectroscopy, and transmission electron microscopy. They were employed as additives to the positive electrode of Ni-MH batteries. Working electrodes, with mixtures of commercial nickel hydroxide and nano-nickel hydroxide (0–10 wt.%) as active material, were prepared. Cyclic voltammetry, charge/discharge profiles, and electrochemical impedance spectroscopy studies were carried out to evaluate the electrochemical performance of the nickel electrode, in 7 M KOH electrolyte, at 25 °C. The presence of nano-nickel hydroxide improves the electrochemical behavior of the active material. The electrochemical impedance spectroscopy (EIS) results were analyzed employing a modified version of previously developed physicochemical model that takes into account the main structural and physicochemical parameters that control these systems.










Similar content being viewed by others
References
Liu X, Yu L (2004) Synthesis of nanosized nickel hydroxide by solid-state reaction at room temperature. Mater Lett 58:1327–1330
Liu X, Yu L (2004) Influence of nanosized Ni(OH)2 addition on the electrochemical performance of nickel hydroxide electrode. J Power Sources 128:326–330
Han XJ, Xu P, Xu CQ, Zhao L, Mo ZB, Liu T (2005) Study of the effects of nanometer β-Ni(OH)2 in nickel hydroxide electrodes. Electrochim Acta 50:2763–2769
Kiani MA, Mousavi MF, Ghasemi S (2010) Size effect investigation on battery performance: comparison between micro- and nano-particles of β-Ni(OH)2 as nickel battery cathode material. J Power Sources 195:5794–5800
Jiang Q, Zhang SH, Li JC (2004) Grain size-dependent diffusion activation energy in nanomaterials. Solid State Commun 130:581–584
Balaya P, Bhattacharyya AJ, Jamnik J, Zhukovskii YF, Kotomin EA, Maier J (2006) Nano-ionics in the context of lithium batteries. J Power Sources 159:171–178
Bruce PG, Scrosati B, Tarascon J-M (2008) Nanomaterials for rechargeable lithium batteries. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Angew Chem Int Ed 47:2930–2946
Ortiz MG, Real SG, Castro EB (2014) Preparation and characterization of positive electrode of Ni-MH batteries with cobalt additives. Int J Hydrog Energy 39:8661–8666
Provazi K, Giz MJ, Dall’ Antonia LH, Córdoba de Torresi SI (2001) The effect of Cd, Co, and Zn as additives on nickel hydroxide opto-electrochemical behaviour. J Power Sources 102:224–232
Watanabe K, Koseki M, Kumagai N (1996) Effect of cobalt addition to nickel hydroxide as a positive material for rechargeable alkaline batteries. J Power Sources 58:23–28
Ortiz MG, Castro EB, Real SG (2014) Effect of cobalt electroless deposition on nickel hydroxide electrodes. Int J Hydrog Energy 39:6006–6012
Martins PR, Araújo Parussulo AL, Hiroshi Toma S, Rocha MA, Eisi Toma H, Arak K (2012) Highly stabilized alpha-NiCo(OH)2 nanomaterials for high performance device application. J Power Sources 218:1–4
Ortiz MG, Castro EB, Real SG (2016) Electrochemical characterization of MWCNT/Ni(OH)2 composites as cathode materials. J Solid State Electrochem 20(4):1029–1036
Liu B, XY W, HT Y, YS Z, DY S, ZX Z (1999) Physical and electrochemical characteristics of aluminium-substituted nickel hydroxide. J Appl Electrochem 29:853–858
Hu WK, Gao XP, Geng MM, Gong ZX, Noréus D (2005) Synthesis of CoOOH nanorods and application as coating materials of nickel hydroxide for high temperature Ni-MH cells. J Phys Chem B 109:5392–5394
Li WY, Zhang S, Chen J (2005) Synthesis, characterization, and electrochemical application of Ca(OH)2-, Co(OH)2-, and Y(OH)3-coated Ni(OH)2 tubes. J Phys Chem B 109:14025–14032
Jiao Q-Z, Tian Z-L, Zhao Y (2007) Preparation of nickel hydroxide nanorods/nanotubes and microscopic nanoring under hydrothermal conditions. J Nanopart Res 9:519–522
Yang L-X, Zhu Y-J, Tong H, Liang Z-H, Liang L, Zhang L (2007) Hydrothermal synthesis of nickel hydroxide nanostructures in mixed solvents of water and alcohol. J Solid State Chem 180:2095–2101
Kong X, Liu X, He Y, Zhang D, Wang X, Li Y (2007) Hydrothermal synthesis of β-nickel hydroxide microspheres with flakelike nanostructures and their electrochemical properties. Mater Chem Phys 106:375–378
Castro EB, Cuscueta DJ, Milocco RH, Ghilarducci AA, Salva HR (2010) An EIS based study of a Ni-MH battery prototype. Modeling and identification analysis. Int J Hydrog Energy 35:5991–5998
Ortiz MG, Becker D, Garaventta G, Visintin A, Castro EB, Real SG (2011) Dynamic monitoring of structural changes in nickel hydroxide electrodes during discharge in batteries. Electrochim Acta 5:7946–7954
Ortiz MG, Castro EB, Real SG (2012) The cobalt content effect on the electrochemical behavior of nickel hydroxide electrodes. Int J Hydrog Energy 37:10365–10370
Li Y, Yao J, Zhu Y, Zou Z, Wang H (2012) Synthesis and electrochemical performance of mixed phase α/β nickel hydroxide. J Power Sources 203:177–183
Ramesh TN, Vishnu Kamath P (2008) The effect of stacking faults on the electrochemical performance of nickel hydroxide electrodes. Mater Res Bull 43:2827–2832
Delmas C, Tessier C (1997) Stacking faults in the structure of nickel hydroxide: a rationale of its high electrochemical activity. J Mater Chem 7:1439–1443
Tessier C, Guerlou-Demourgues L, Faure C, Basterreix M, Nabias G, Delmas C (2000) Structural and textural evolution of zinc-substituted nickel hydroxide electrode materials upon ageing in KOH and upon redox cycling. Solid State Ionics 133:11–23
Ramesh TN (2009) Crystallite size effects in stacking faulted nickel hydroxide and its electrochemical behaviour. Mater Chem Phys 114:618–623
Guerlou-Demourgues L, Tessier C, Bernard P, Delmas C (2004) Influence of substituted zinc on stacking faults in nickel hydroxide. J Mater Chem 14:2649–2654
Bernard MC, Cortes R, Keddam M, Takenouti H, Bernard P, Senyarich S (1996) Structural defects and electrochemical reactivity of β-Ni(OH)2. J Power Sources 63:247–254
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978–982
Nathira Begum S, Muralidharan VS, Ahmed Basha C (2009) The influences of some additives on electrochemical behaviour of nickel electrodes. Int J Hydrog Energy 34:1548–1555
Wang X, Luo H, Parkhutik PV, Millan A-C, Matveeva E (2003) Studies of the performance of nanostructural multiphase nickel hydroxide. J Power Sources 115:153–160
Shruthi B, Bheema Raju V, Madhu BJ (2015) Synthesis, spectroscopic and electrochemical performance of pasted β-nickel hydroxide electrode in alkaline electrolyte. Spectrochim Acta A Mol Biomol Spectrosc 135:683–689
De Levie R (1967) In: Delahay P (ed) Advances in electrochemistry and electrochemistry engineering, 6. Interscience, NY, pp. 329–361
Ho C, Raistrick ID, Huggins RA (1980) Application of A-C techniques to the study of lithium diffusion in tungsten trioxide thin films. J Electrochem Society 127:343–350
Jacobsen T, West K (1995) Diffusion impedance in planar, cylindrical and spherical symmetry. Electrochim Acta 40:255–262
Barsoukov E, Macdonald JR (2005) Impedance spectroscopy: theory, experiment, and applications, 2nd edn N.J. Wiley-Interscience, Hoboken
Arico AS, Bruce P, Scrosati B, Tarascon J-M, Van Schalkwijc W (2004) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377
Acknowledgments
The authors would like to acknowledge the financial support from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Universidad Tecnológica Nacional (UTN).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Castro E.B. passed away on 18th February 2013.
Rights and permissions
About this article
Cite this article
Real, S.G., Ortiz, M.G. & Castro, E.B. Electrochemical characterization of nickel hydroxide nanomaterials as electrodes for Ni-MH batteries. J Solid State Electrochem 21, 233–241 (2017). https://doi.org/10.1007/s10008-016-3355-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3355-8