Abstract
Properties of CF x /Li and CF x /Na cells were examined while using galvanostatic charging/discharging, electrochemical impedance spectroscopy and scanning electron microscopy (SEM). The capacity during the first cycle was as high as ca. 1000 mAh g−1. Such an electrode is suitable for primary CF x /Li and CF x /Na batteries. SEM images of CF x cathode showed that during discharging it was transformed into amorphous carbon and LiF or NaF crystals (of diameter of ca. 5–20 μm). These systems (C + LiF or C + NaF) cannot be reversibly converted back into CF x /Li or CF x /Na, respectively. Exchange current densities are between 10−7 Acm−2 and 10−9 Acm−2 when working with LiPF6 and NaPF6 electrolytes (1.12 × 10−7 Acm−2 and 6.82 × 10−9 Acm−2, respectively). Those values are low and indicate that the charge transfer process may be the rate-determining step. Activation energies for the charge transfer process were 57 and 72 kJ mol−1 for CF x /LiPF6 and CF x /NaPF6 systems, respectively. Higher activation energy barrier for the CF/Na+ + e− → C + NaF reaction results in lower observed exchange current density in comparison to the system with lithium ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Li-ion batteries, lithium metal anode is replaced by lithium-intercalated carbons (LiC x ) due to the irreversible behavior of this metal. Hence, battery contains lithium in the form of intercalated compounds and electrolyte. Total amount of lithium in seawater is high (2.4 × 1011 tons) but the concentration is constant and low: 2.5 × 10−5 mol l−1 (0.173 mg l−1) in contrast to sodium (0.468 mol l−1 or 10.77 g l−1) [1]. Therefore, lithium resources are brine (Argentina, Chile, and the USA) and minerals whereas sodium can be electrochemically recovered from abounded sodium chloride. In addition, lake brine can contain more magnesium than lithium (for example in the ratio of 40:1) [2]. As a result, sodium is cheaper in comparison to lithium—US$5000 and US$150 for 1 ton of Li2CO3 and Na2CO3, respectively [3]. While carbon lithium-intercalated anodes work reversibly for ca. 103 cycles [4–10] the carbon-sodium system does not show such a promising reversible capacity [11–16]. Therefore, Li-ion systems are fully commercialized but Na-ion batteries are still under research [3, 12, 17–37]. On the other hand, there is a demand for primary power sources of high energy density. Both metallic lithium and sodium are sufficient anode materials for such batteries due to their capacity (Li: 3829 mAh g−1 and Na: 1165 mAh g−1) [3]. Due to much lower sodium price (in comparison to lithium), it seems to be a more attractive candidate for metallic anode. Another problem is a selection of a suitable cathode of the capacity comparable to that characteristic of Li or Na anodes. Many cathodic systems were selected, but their capacity was usually of the order of 100 mAh g−1 [11]. Fluorinated graphite, CF x , was synthesized in 1934 and it was shown that it can be used as a cathode and even Li/CF x battery was commercialized [38]. Then different fluorinated CF x materials of different fluorination level were prepared [39, 40]. Discharge capacities of different CF x materials are reported to be between 600 and 900 mAh g−1 [40, 41] with open circuit potential against metallic lithium between 2.4 and 3.5 V, depending on CF x material and electrolyte [39, 42] and energy density of 2000 Wh kg−1 [43]. The theoretical capacity of CF x is 865 mAh g−1 for x = 1 and decreases as the x value increases [43]. It was demonstrated that CF x materials of high fluorination level did not show enhanced capacity, as CF2 groups were electrochemically inactive [40]. While Li/CF x battery was extensively studied [44–54], to our knowledge the electrochemical properties of Na/CF x system were reported once in 2014 [55]. The papers were focused on systems capacity and mechanism of electrochemical and chemical processes. Nonetheless, power of electrochemical systems depends on kinetic limits. The general aim of the present study has been kinetic characterization of Na/CF x system and its comparison to Li/CF x battery.
Experimental
Materials
Sodium sticks (Aldrich), lithium foil (0.75 mm thick, Aldrich), vinylene carbonate (VC, Aldrich), ethylene carbonate (EC, Aldrich), propylene carbonate (PC, Aldrich), dimethyl carbonate (DMC, Aldrich) lithium hexafluorophosphate (LiPF6, Aldrich), sodium hexafluorophosphate (NaPF6, Aldrich), graphite fluoride (CF x , ACS Material), carbon black (CB, Alfa Aesar), poly(vinylidene fluoride) (PVdF, Fluka), and N-methyl-2-pyrrolidinone (NMP, Fluka) were used as received from suppliers.
Vinylene carbonate was stored at a max temperature of 8 °C to prevent its from spontaneous polymerization. Liquid electrolytes (90 wt% of 1 M LiPF6 or 1 M NaPF6 in PC-DMC (1:1 wt) + 10 wt% of VC) were obtained by dissolution of solid LiPF6 or NaPF6 salt in liquid mixture PC-DMC (90 wt%) + VC (10 wt%). Solutions of electrolytes were prepared and cells were assembled in a glove box in the dry argon atmosphere.
CF x electrode was prepared with a composition of 80 wt% CF x , 10 wt% carbon black (CB) conductive additive and 10 wt% polyvinylidene fluoride (PVDF) binder by mixing calculated amounts of CF x , CB and PVDF with 1-methyl-2pyrrolidone (NMP) solvent. The suspension of solid components in NMP was cast on Au current collector. After solvent (NMP) evaporation at 120 °C in a vacuum, a layer of the carbon electrode was formed containing the active material (CF x ), electronic conductor (CB), and the binder (PVdF). The electrode contained typically 3–6 mg of the fluoride graphite. A round-shaped metallic sodium or lithium counter electrode were formed from stick metallic sodium and cut-off from the metallic lithium foil, respectively. The surface area of the Li and Na electrodes was 1.27 cm2.
Measurements
The CF x (CF x + CB + PVdF) electrodes were separated from sodium or lithium by glass microfiber separator (GF/A, Whatman) soaked with the electrolyte. CF x |electrolyte|sodium and CF x |electrolyte|lithium systems were placed in an adapted 0.5″ Swagelok® connecting tube. Electrochemical properties of cells were characterized using electrochemical impedance spectroscopy (EIS) and galvanostatic charging/discharging tests. Galvanostatic curves of charging/discharging and impedance spectra (frequency range of 100 kHz–10 mHz, at open circuit potential and amplitude of 10 mV) were obtained using a frequency response analyzer (multichannel Interface 1000, Potentiostat/Galvanostat system, Gamry, USA). Deconvolution of spectra was performed with the Gamry software. The morphology of graphite fluoride electrodes (pristine and after intercalation of sodium and lithium) were analyzed using electron scanning microscope (TESCAN Vega 5153, Czech Republic). BET surface of pristine CF x material was determined with Autosorb iQ apparatus (Quantochrome Instruments, UK).
Results and discussion
Galvanostatic charging/discharging
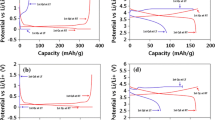
CF x cathode was tested galvanostatically in CF x |1 M LiPF6 PC-DMC 10 % VC|Li and CF x |1 M NaPF6 PC-DMC 10 % VC|Na systems. As a matter of fact, in all experiments the mass of the lithium or sodium anodes was much higher (ca. 40 mg) or electrochemically equivalent to the mass of the cathode. Open circuit potential of the cells measured immediately after their assembling was ca. 3.3 V. Figure 1 shows the discharging/charging curves for both systems with current densities of 50–5 mA g−1. Voltage versus product of time t and current I (usually assumed to reflect the electrode capacity q) in both cases showed two distinct plateaus. In the case of CF x |Li cell the first plateau occurs at voltages of 2.34–2.1 V and the second 1.6–1.36 V, while CF x |Na system at ca. 2.0 V (1.99–1.79 V) and 1.3–1.05 V. Cells containing electrolytes without VC showed the second plateau at lower voltage of ca. 0.5 V. This is why the experiments were performed using electrolytes containing VC as an additive. Voltage versus q curves for the systems under study were published previously for voltages not lower than ca. 2 V and the cathode material was obtained from different source [55]. This makes both systems difficult to compare. However, the initial capacity of the first plateau of the Na/CF x was reported to be ca. 1000 mAh g−1 [55], to decrease to ca. 786 and 409 mAh g−1 after the second and sixth cycle, respectively (at a current density of 200 mAh g−1, discharge to 1.5 V). This suggests rather irreversible nature of the system. It was stated that “obviously, Na/CF x batteries should be further improved [55].” Tested cells in this work showed two distinct plateaus within the q = I∙t value of ca. 1000 mAh g−1, including the second plateau with q at a lower level ca. three times, but energy delivered is even lower as the potential of the second plateau is ca. 1 V (and not 2 V). Poor reversibility and capacity fading during cycling within the first plateau indicate that CF x |Na systems are rather good candidates for primary batteries and not rechargeable ones. Free enthalpy associated with CF x |Na+ cathode discharging till the end of the first plateau was ca. 2 V × 600 mAh g−1 ≈ 4.32 kJ g−1 while the corresponding value for the second plateau is only ca. 1.3 V × 211 mAh g−1 = 0.99 kJ g−1. Integration of I = f(t) curves shown in Fig. 1 gave higher overall capacity (ca. 1000 mAh g−1) higher amount of energy delivered to be 6.06 and 5.79 kJ g−1 for CF x |Li and CF x |Na primary batteries (expressed versus CF x mass), respectively. At lower current regimes capacitance calculated as q = It is somewhat higher (ca. 7 %). Discharge curves and CF x capacities for CF x |Na system working with 1 M LiPF6 in EC + DMC (1:1) + 10 wt% VC were similar to those obtained for the electrolyte containing PC instead of EC. Potential recorded during Li/CF x or Na/CF x discharging is a mixed potential of electrodefluorination of CF x material to carbon and F− anions (the first plateau) and electrolyte decomposition [56]. After electrochemical de-fluorination, the surface inactive CF2 groups are eliminated to form a more electronic and ionic conductive surface [57]. Figure 2 shows SEM images of pristine CF x electrode (Fig. 2a) and then after its discharging (Fig. 2b: CF x |Li and Fig. 2c: CF x |Na). It can be seen that the pristine CF x material consists of fluorinated graphite particles, which were transformed during the electrode reaction into amorphous carbon and LiF or NaF crystals of diameter of ca. 5–20 μm.
Impedance studies
Figure 3 shows electrochemical impedance spectra (EIS) of CF x electrode taken after CF x |Li and CF x |Na systems assembling (Fig. 3a, b). Spectra consist of two parts: a semicircle and a straight line at lower frequencies. Corresponding spectra for discharged systems are shown in Fig. 3c, d. It can be seen that impedance decreased considerably. This is probably due to conversion of CF x into solid C/LiF or C/NaF. In addition, lithium and sodium anodes without CF x counter electrode do not show such high impedances (Fig. 3e, f). This suggests that CF x material is responsible for the high total impedance of CF x /Li and CF x /Na systems. Goodness of the fit was close to 10−4–10−5 (Fig. 4 shows examples of the fits overlaid with the experimental data). On the other hand, total impedance of both systems decreased considerably with temperature increase (Fig. 5). Curves shown in Fig. 5 were deconvoluted taking into account two time constants RC, due to the SEI layer (R SEI, C SEI) and the charge transfer process which occurs at the double layer formed between SEI and the anode (R ct, C dl). The straight line at the low frequency section is due to the Li+ and Li diffusion. Therefore, the equivalent circuit consisted of electrolyte resistance (R el) in series with two time constants (R and C in parallel: R SEI, C SEI and R ct, C ct) and Warburg element Z W.
The value of the charge transfer resistance at 25 °C estimated from the deconvolution procedure was 57 Ω for CF x /Li and as high as 862 Ω for CF x /Na. Determined R ct resistances can be expressed versus real surface area of cathode material S BET = 93.6 m2 g−1, estimated from BET measurement. The mass of CF x cathode material used in impedance experiments was 4.32 and 4.72 mg in CF x /Li and CF x /Na cells, respectively. This leads to electrodes real surface area A and corresponding R ct∙A values expressed in Ω cm2 (Table 1). Charge transfer resistances may be converted into exchange current densities j o (1):
Exchange current densities listed in Table 1 are of the order of 10−7 Acm−2 when working with LiPF6 (1.12 × 10−7 Acm−2) and ca. two orders of magnitude lower for NaPF6 electrolyte (6.82 × 10−9 Acm−2). Those values are low and indicate that the charge transfer process may be the rate-determining step. In addition, the CF x material is resistive; however, even during a short time of discharge, it is coated with conductive carbon particles [58, 59]. It has been shown for the Li/CF x system that at low rates of discharge (through a fixed resistive load attached to each tested cell) the system obeys Tafel kinetics and transport properties are of negligible importance [58, 59]. Consequently, this observation suggested that the kinetic rate constant was very small [58].
The resistance R ct, obtained at different temperatures, gave lnR −1 = f(T −1) straight line with a slope indicating activation energy E # of the overall process in the cell during its discharging. Obtained activation energies for the charge transfer process were 57 and 72 kJ mol−1 for CF x /LiPF6 and CF x /NaPF6 systems, respectively. Higher activation energy barrier for the CF/Na+ + e− → C + NaF reaction results in lower observed exchange current density in comparison to the system with lithium ions.
Deconvolution of impedance spectra of Li/Li and Na/Na systems, without CF x cathode (shown in Fig. 3e, f) gave charge transfer resistance values for the Li/Li+ (48 Ω) and (201 Ω). Taking into account anodes surface area of 1.27 cm2, resulting exchange current densities are 0.41 and 0.10 mA cm−2 for Li/Li+ and Na/Na+, respectively. Charge transfer process for sodium is slower in comparison to that characteristic of lithium; however, 3–4 orders of magnitude faster in comparison to that characteristic of CF x cathode. Kinetics of Li/Li+ and Na/Na+ was also investigated in ionic liquids [60–65]. In the literature, the charge transfer reaction is described by exchange current density, j o, rate constant k o or corresponding resistance R ct, which are mutually related. Cyclic voltammetry experiments on Pt and Ni electrodes gave similar value of charge transfer rate constant k o ∼ 10−5 cm s−1 for both Li/Li+ [60, 62] and Na/Na+ [61] systems. In the case of metallic lithium and sodium electrodes a protective layer of the solid electrolyte interphase (SEI) is formed. In such a case, the charge transfer reaction takes place at the interphase between two solids: Li/SEI or Na/SEI. This makes recalculation of k o into R ct or j o difficult.
Conclusions
Cyclability of the CF x cathode is generally poor. However, the capacity at the first cycle is as high as ca. 1000 mAh g−1. Such an electrode is suitable for primary CF x /Li and CF x /Na batteries. During discharging, the CF x cathode was transformed into amorphous carbon and LiF or NaF crystals of diameter of ca. 5–20 μm. These systems (C + LiF or C + NaF) cannot be reversibly converted back into CF x /Li or CF x /Na, respectively. Exchange current densities are of the order of 10−7 Acm−2–10−9 Acm−2 when working with LiPF6 and NaPF6 electrolytes (1.12 × 10−7 Acm−2 and 6.82 × 10−9 Acm−2, respectively). Those values are low and indicate that the charge transfer process may be the rate-determining step. Activation energies for the charge transfer process were 57 kJ mol−1 and 72 kJ mol−1 for CF x /LiPF6 and CF x /NaPF6 systems, respectively. Higher activation energy barrier for the CF/Na+ + e− → C + NaF reaction results in lower observed exchange current density in comparison to the system with lithium ions.
References
Schwochau K (1984) Extraction of metals from sea water. Top Curr Chem 124:91–133
Liu X, Chen X, He L, Zhao Z (2015) Study on extraction of lithium from salt lake brine by membrane electrolysis. Desalination 376:35–40
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-Ion Batteries. Adv Funct Mater 23:947–958
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195:2419–2430
Goriparti S, Miele E, De Angelis F, Di Fabrizio E, Proietti Zaccaria E, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources 257:421–443
Xu K (2014) Electrolytes and interphases in Li-ion batteries and beyond. Chem Rev 114:11503–11618
Nitta N, Wu F, Lee JT, Yushin G (2014) Li-ion battery materials: present and future. Mater Today 18:252–264
Roy P, Srivastava SK (2015) Nanostructured anode materials for lithium ion batteries. J Mater Chem A 3:2454–2484
Diouf B, Pode R (2015) Potential of lithium-ion batteries in renewable energy. Renew Energy 76:375–380
Marom R, Amalraj SF, Leifer N, Jacob D, Aurbach D (2011) A review of advanced and practical lithium battery materials. J Mater Chem 21:9938–9954
Xiang X, Zhang K, Chen J (2015) Recent advances and prospects of cathode materials for sodium-ion batteries. Adv Mater 27:5343–5364
Go D-Y, Park J, Noh P-J, Cho G-B, Ryu H-S, Nam T-H et al (2014) Electrochemical properties of monolithic nickel sulfide electrodes for use in sodium batteries. Mater Res Bull 58:190–194
Park J, Kim J-S, Park J-W, Nam T-H, Kim K-W, Ahn J-H et al (2013) Discharge mechanism of MoS2 for sodium ion battery: electrochemical measurements and characterization. Electrochim Acta 92:427–432
Ryu H, Kim T, Kim K, Ahn JH, Nam T, Wang G et al (2011) Discharge reaction mechanism of room-temperature sodium-sulfur battery with tetraethylene glycol dimethyl ether liquid electrolyte. J Power Sources 196:5186–5190
Wongittharom N, Wang C, Wang Y, Yang C, Chang J (2014) Ionic liquid electrolytes with various sodium solutes for rechargeable Na/NaFePO4 batteries operated at elevated temperatures. ACS Appl Mater Interfaces 6:17564–17570
Wang L, Lu Y, Liu J, Xu M, Cheng J, Zhang D (2013) A superior low-cost cathode for a Na-ion battery. Angew Chemie-Int Ed 52:1964–1967
Xie X, Ao Z, Su D, Zhang J, Wang G (2015) MoS2/graphene composite anodes with enhanced performance for sodium-ion batteries: the role of the two-dimensional heterointerface. Adv Funct Mater 25:1393–1403
Deng W, Liang X, Wu X, Qian J, Cao Y, Ai X et al (2013) A low cost, all-organic Na-ion battery based on polymeric cathode and anode. Sci Rep 3:1–6
Billaud J, Cle J, Armstrong AR, Rozier P, Grey CP, Bruce PG (2014) β-NaMnO2: a high-performance cathode for sodium-ion batteries. J Am Chem Soc 136:17243–17248
Kumar PR, Jung YH, Kim DK (2015) High performance of MoS2 microflowers with a water-based binder as an anode for Na-ion batteries. RSC Adv 5:79845–79851
Yang YY, Yang X, Zhang Y, Hou H, Jing M, Zhu Y et al (2015) Cathodically induced antimony for rechargeable Li-ion and Na-ion batteries: the influences of hexagonal and amorphous phase. J Power Sources 282:358–367
Yuan D, He W, Pei F, Wu F, Wu Y, Qian J et al (2013) Synthesis and electrochemical behaviors of layered Na0.67[Mn0.65Co0.2Ni0.15]O2 microflakes as a stable cathode material for sodium-ion batteries. J Mater Chem A 1:3895–3899
Shen Y, Wang X, Hu H, Jiang M, Yang X, Shu H (2015) A graphene loading heterogeneous hydrated forms iron based fluoride nanocomposite as novel and high-capacity cathode material for lithium/sodium ion batteries. J Power Sources 283:204–210
Barpanda P, Lu J, Ye T, Kajiyama M, Chung S-C, Yabuuchi N et al (2013) A layer-structured Na2CoP2O7 pyrophosphate cathode for sodium-ion batteries. RSC Adv 3:3857–3860
Lu Y, Wang L, Cheng J, Goodenough JB (2012) Prussian blue: a new framework of electrode materials for sodium batteries. Chem Commun 48:6544–6546
Kang YH, Liu Y, Cao K, Zhao Y, Jiao L, Wang Y et al (2015) Update on anode materials for Na-ion batteries. J Mater Chem A 3:17899–17913
Kundu D, Tripathi R, Popov G, Makahnouk WRM, Nazar LF (2015) Synthesis, structure and Na-ion migration in Na4NiP2O7F2: a prospective high voltage positive electrode material for the Na-ion battery. Chem Mater 27:885–891
Han MH, Gonzalo E, Singh G, Rojo T (2015) A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries. Energy Environ Sci 8:81–102
Kim Y, Ha K-H, Oh SM, Lee KT (2014) High-capacity anode materials for sodium-ion batteries. Chem-A Eur J 20:11980–11992
Yabuuchi N, Kubota K, Dahbi M, Komaba S (2014) Research development on sodium-ion batteries. Chem Rev 114:11636–11682
Palomares V, Casas-Cabanas M, Castillo-Martínez E, Han MH, Rojo T (2013) Update on Na-based battery materials. A growing research path. Energy Environ Sci 6:2312–2337
Hong SY, Kim Y, Park Y, Choi A, Choi N-S, Lee KT (2013) Charge carriers in rechargeable batteries: Na ions vs. Li ions. Energy Environ Sci 6:2067–2081
Bommier C, Ji X (2015) Recent development on anodes for Na-ion batteries. Isr J Chem 55:486–507
Ellis BL, Nazar LF (2012) Sodium and sodium-ion energy storage batteries. Curr Opin Solid State Mater Sci 16:168–177
Pan H, Hu Y-S, Chen L (2013) Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ Sci 6:2338–2360
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5:5884–5901
Dahbi M, Yabuuchi N, Kubota K, Tokiwa K, Komaba S (2014) Negative electrodes for Na-ion batteries. Phys Chem Chem Phys 16:15007–15028
Nakajama T (1999) Carbon-fluorine compounds as battery materials. J Fluorine Chem 100:57–61
Guerin K, Dubois M, Hamwi A (2006) Electrochemical discharge mechanism of fluorinated graphite used as electrode in primary lithium batteries. J Phys Chem Solids 67:1173–1177
Giraudet J, Delabarre C, Guerin K, Dubois M, Masin F, Hamwi A (2006) Comparative performances for primary lithium batteries of some covalent and semi-covalent graphite fluorides. J Power Sources 158:1365–1372
Delabarre C, Dubois M, Giraudet J, Guerin K, Hamwi A (2006) Electrochemical performance of low temperature fluorinated graphites used as cathode in primary lithium batteries. Carbon 44:2543–2548
Zhang Q, D’Astorg S, Xiao P, Zhang X, Lu L (2010) Carbon-coated fluorinated graphite for high energy and high power densities primary lithium batteries. J Power Sources 195:2914–2917
Zhang Q, Takeuchi KJ, Takeuchi ES, Marschilok AC (2015) Progress towards high-power Li/CFx batteries: electrode architectures using carbon nanotubes with CFx. Chem Phys 17:22504–22518
Hamwi A, Guérin K, Dubois M (2005) Fluorine-intercalated graphite for lithium batteries. In: Nakajima T, Groult H (eds) Fluorinated materials for energy conversion. Elsevier Ltd, Oxford, pp 369–395
Whitacre J, Yazami R, Hamwi A, Smart MC, Bennett W, Prakash GKS, Miller T, Bugga R (2006) Low operational temperature Li–CF x batteries using cathodes containing sub-fluorinated graphitic materials. J Power Sources 160:577–584
Ahmad Y, Dubois M, Guérin K, Hamwi A, Zhang W (2015) Pushing the theoretical limit of Li–CF x batteries using fluorinated nanostructured carbon nanodiscs. Carbon 94:1061–1070
Guérin K, Yazami R, Hamwi A (2014) Hybrid-type graphite fluoride as cathode material in primary lithium batteries. Electrochem Solid St 7:A159–A162
Delabarre C, Dubois M, Giraudet J, Guerin K, Yazami R, Hamwi A (2007) Comparative electrochemical study of low temperature fluorinated graphites used as cathode in primary lithium batteries. T Electrochem Soc 3:153–163
Dubois M, Guérina K, Zhanga W, Ahmada Y, Hamwi A, Fawal Z, Kharbache H, Masin F (2012) Tuning the discharge potential of fluorinated carbon used as electrode in primary lithium battery. Electrochim Acta 59:485–491
Amatucci GG, Pereira N (2007) Fluoride based electrode materials for advanced energy storage devices. J Fluorine Chem 128:243–262
Read J, Collins E, Piekarski B, Zhang S (2011) LiF formation and cathode swelling in the Li/CF x battery. J Electrochem Soc 158:A504–A510
Zhang SS, Foster D, Wolfenstine J, Read J (2009) Electrochemical characteristic and discharge mechanism of a primary Li/CF x cell. J Power Sources 187:233–237
Lam P, Yazami R (2006) Physical characteristics and rate performance of (CF x ) n (0.33 < x <0.66) in lithium batteries. J Power Sources 153:354–359
Yazami R, Hamwi A, Guérin K, Ozawa Y, Dubois M, Giraudet J, Masin F (2007) Fluorinated carbon nanofibres for high energy and high power densities primary lithium batteries. Electrochem Commun 9:1850–1855
Liu W, Li H, Xie J-Y, Fu Z-W (2014) Rechargeable room-temperature CF x -sodium battery. ACS Appl Mater Interface 6:2209–2112
Hunger HF, Ellison JE (1975) Rate capability and electrochemical stability of carbon fluoride compounds in organic electrolytes. J Electrochem Soc 122:1288–1291
Dai Y, Cai S, Wu L, Yang W, Xie J, Wen J, Zheng J-C, Zhu Y (2014) Surface modified cathode material for ultrafast discharge and high energy density. J Mater Chem A 2:20896–20901
Davis S, Takeuchi ES, Tiedemann W, Newman J (2007) Simulation of the Li-CF x system. J Electrochem Soc 154:A477–A480
Davis S, Takeuchi ES, Tiedemann W, Newman J (2008) Simulation of pulse discharge of the Li-CF x system. J Electrochem Soc 155:A24–A28
Wibowo R, Ward Jones SE, Compton R (2009) Kinetic and thermodynamic parameters of the Li/Li + couple in the room temperature ionic liquid N-butyl-N-metylpyrrolidinium bis(trifluoromethylsulfonyl) imide in the temperature range 298–318 K: a theoretical and experimental study using Pt and Ni electrodes. J Phys Chem B113:12293–12298
Wibowo R, Aldous L, Rogers EI, Ward Jones SE, Compton RG (2010) A study the Na/Na+ redox couple in some room temperature ionic liquids. J Phys Chem C114:3618–3626
Wibowo R, Ward Jones SE, Compton RG (2010) Investigating the electrode kinetics of the Li/Li+ couple in a wide range of room temperature ionic liquids at 298 K. J Chem Eng Data 55:1374–1376
Lewandowski A, Swiderska-Mocek A, Waliszewski L, Galinski M (2012) Lithium redox behaviour in N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl) imide room temperature ionic liquid. J Power Sources 197:292–296
Lewandowski A, Biegun M, Galinski M (2012) Kinetics of Li+ reduction in 1-methyl-3-propylpiperidinium bis(trifluoromethylsulfonyl) imide room temperature ionic liquid. Electrochim Acta 63:204–208
Lewandowski A, Biegun M, Galinski M, Swiderska-Mocek A (2013) Kinetic analysis of Li/Li+ interphase in an ionic liquid electrolyte. J Appl Electrochem 43:367–374
Acknowledgments
Support of the grant NCN UMO/2013/09/B/ST4/00107 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lewandowski, A., Jakobczyk, P. Kinetics of Na|CF x and Li|CF x systems. J Solid State Electrochem 20, 3367–3373 (2016). https://doi.org/10.1007/s10008-016-3305-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3305-5