Abstract
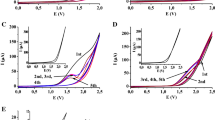
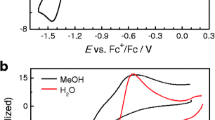
Cyclic voltammetry of tannic acid mixture (TAM) in water, dimethylsulfoxide (DMSO), and acetonitrile (ACN) indicates the highest oxidation potential for TAM in ACN, followed by the slightly lowered potential in DMSO and a strongly shifted oxidation potential in water solutions, confirming its pH-dependent redox behavior. In situ EPR and UV–Vis spectroelectrochemical experiments were performed to follow the oxidation reactions of TAM in protic and aprotic media. The formation of an unstable semiquinone anion radical formed upon anodic oxidation of TAM was proved by in situ EPR spectroelectrochemistry both in DMSO and water solutions. The quantum chemical calculations of the model pyrogallol derivatives and tannic acid molecules with four and ten galloyl moieties estimated the role of the spatial hydrogen bonds on the proton affinities and suggested the possible interpretation of experimentally detected redox and spectroelectrochemical behaviors.








Similar content being viewed by others
References
Chung KT, Wong TY, Wei CI, Huang YW, Lin Y (1998) Crit Rev Food Sci Nutr 38:421–464
Hagerman AE, Riedl KM, Rice RE (1999) Basic Life Sci 66:495–505
Jourdes M, Pouysegu L, Deffieux D, Teissedre P-L, Quideau S (2013) Hydrolyzable tannins: Gallotannins and ellagitannins. In: Ramawat KG, Merillon JM (eds) Natural products: Phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Springer, Berlin, pp 1975–2010
Cao Y, Himmeldirk KB, Qian Y, Ren Y, Malki A, Chen X (2014) J Nat Med 68:465–472
Zhang J, Li L, Kim SH, Hagerman AE, Lü J (2009) Pharm Res 26:2066–2080
Qu XJ, Zhou J (2002) Chin J Anal Chem 30:192
Lu S (2004) Russ J Electrochem 40:750–754
Wan H, Zou Q, Yan R, Zhao F, Zeng B (2007) Microchim Acta 159:109–115
Xu L, He N, Du J, Deng Y (2008) Electrochem Commun 10:1657–1660
Hung YT, Chen PC, Chen RLC, Cheng TJ (2008) Sensor Actuat B - Chem 130:135–140
Xu L, He N, Du J, Deng Y, Li Z, Wang T (2009) Anal Chim Acta 634:49–53
Raj MA, Revin SB, John SA (2013) Bioelectrochemistry 89:1–10
Vu DL, Ertek B, Červenka L, Dilgin Y (2013) Int J Electrochem Sci 8:9278–9286
Lemanska K, Szymusiak H, Tyrakowska B, Zielinski R, Soffers EMF, Rietjens IMCM (2001) Free Radic Biol Med 31:869
Žemlička L, Fodran P, Lukeš V, Vagánek A, Slováková M, Staško A, Dubaj T, Liptaj T, Karabin M, Bírošová L, Rapta P (2014) Monatsh Chem 145:1307–1318
Klein E, Rimarčík J, Lukeš V (2009) Acta Chim Slovaca 2:37–51
Rimarčík J, Lukeš V, Klein E, Ilčin M (2010) J Mol Struct (THEOCHEM) 952:25–30
Rimarčík J, Lukes V, Klein E, Rottmannova L (2011) Comput Theor Chem 967:273–283
Vagánek A, Rimarčík J, Lukeš V, Klein E (2012) Comput Theor Chem 991:192–200
Becke AD (1993) J Chem Phys 98:5648–5652
Grimme S, Ehrlich S, Goerigk L (2011) J Comp Chem 32:1456–1465
Furche F, Ahlrichs R (2002) J Chem Phys 117:7433–7447
Yanai T, Tew D, Handy N (2004) Chem Phys Lett 393:51–57
Cancès E, Mennucci B (1998) J Math Chem 23:309–326
Dapprich S, Komáromi I, Byun KS, Morokuma K, Frisch MJ (1999) J Mol Struct (THEOCHEM) 462:1–21
Martínez L, Andrade R, Birgin EG, Martínez JM (2009) J Comp Chem 30:2157–2164
Rappé AK, Casewit CJ, Colwell KS, Goddard WA III, Skiff WM (1992) J Am Chem Soc 114:10024–10035
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al. (2009) Gaussian, Inc., Wallingford CT
Binkley JS, Pople JA, Hehre WJ (1980) J Am Chem Soc 102:939–947
Molekel 4.3, Flukiger P, Luthi HP, Sortmann S, Weber J (2002) Swiss National Supercomputing Centre, Manno, Switzerland
Vedernikova I, Salahub D, Proynov E (2003) J Mol Struct (THEOCHEM) 663:59–71
Meyer TJ, Huynh MHV, Thorp HH (2007) Angew Chem Int Ed 46:5284–5304
Angel LA, Ervin KM (2006) J Phys Chem A 110:10392–10403
Atkins PW (1998) Physical chemistry, 6th edn. Oxford University Press, Oxford
Fifen JJ, Nsangou M, Dhaouadi Z, Motapon O, Jaidane N (2011) Comp Theor Chem 966:232–243
Rimarčík J, Lukeš V, Klein E, Ilčin M (2010) J Mol Struc 952:25–30
Mejías JA, Lago S (2000) J Chem Phys 113:7306–7316
Acknowledgments
This work was supported by the Scientific Grant Agency of the Slovak Republic (Projects 1/0735/13 and 1/0307/14). We are grateful to the HPC center at the Slovak University of Technology in Bratislava, which is a part of the Slovak Infrastructure of High Performance Computing (SIVVP project, ITMS code 26230120002, funded by the European Region Development Funds, ERDF) for the computational time and resources made available.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Mikhail A. Vorotyntsev in the occasion of his 70th birthday
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 111435 kb)
Rights and permissions
About this article
Cite this article
Lukeš, V., Darvasiová, D., Furdíková, K. et al. Solvent effect on the anodic oxidation of tannic acids: EPR/UV–Vis spectroelectrochemical and DFT theoretical study. J Solid State Electrochem 19, 2533–2544 (2015). https://doi.org/10.1007/s10008-015-2921-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2921-9