Abstract
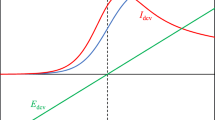
Multi-scan cyclic voltammetry may lead eventually to a repetitive current-versus-potential graph. Here such “ultimate” cyclic voltammograms are investigated mathematically for reversible electrode reactions. The voltammetric shapes, after an infinite number of cycles, are modelled and their characteristics are documented. It is predicted that the approach to truly repetitive behaviour, within an experimentally realistic length of time, requires that a specific condition—balancing the potential scan range to the initial concentrations—be satisfied.







Similar content being viewed by others
Notes
If, for example, \( t=-{\scriptscriptstyle \frac{25}{16}}P,\mathrm{then}\operatorname{Int}\left\{-2t/P\right\}=3\operatorname{and}\mathrm{frac}\left\{-2t/P\right\}={\scriptscriptstyle \frac{1}{8}}. \)
In contradistinction, the lower limit in the companion article [1] is zero.
In a Fourier representation, antisymmetry corresponds to all the coefficients of even harmonic number h equalling zero; that is: a 2 = b 2 = a 4 = b 4 = a 6 = ⋯ = 0.
except, perhaps, for some “this layer” configurations.
also known misleadingly as a “stationary” state
References
Oldham KB (2013) Multi-scan reversible cyclic voltammetry: is the ultimate condition approached and, if so, how fast? J Solid State Electrochem. doi:10.1007/s10008-013-2175-3
Noel M, Vasu KI (1990) Cyclic voltammetry and frontiers of electrochemistry. Aspect, London
Grosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanisms. VCH, New York
Compton RG, Banks CE (2007) Understanding voltammetry. World Scientific, Singapore
Marken F, Neudeck A, Bond AM (2012) In: Scholz F (ed) Electrochemical methods, 2nd edn. Heidelberg, Springer
Myland JC, Oldham KB (1983) An analytical expression for the current-voltage relationship during reversible cyclic voltammetry. J Electroanal Chem 153:43
Keightley AM, Myland JC, Oldham KB, Symons PG (1992) Reversible cyclic voltammetry in the presence of product. J Electroanal Chem 322:25
Oldham KB, Feldberg SW (1999) The principle of unchanging total concentration and its implications for modeling unsupported transient voltammetry. J Phys Chem B 103:1699
Bard AJ, Inzelt G, Scholz F(eds) (2012) Electrochemical Dictionary 2nd edn. Springer, Berlin, p 362
Oldham KB (1996) Tables of semiintegrals. J Electroanal Chem 430:1
Oldham K, Myland J, Spanier J (2000) An atlas of functions, 2nd edn. Springer, New York, Chap 64
Wolfram S (1999) The Mathematica® Book, 4th edn. Cambridge University Press, Cambridge
Oldham KB, Myland JC, Bond AM (2012) Electrochemical Science and Technology. Wiley, Chichester, equ 16:33
Bard AJ, Faulkner LR (2001) Electrochemical Methods, 2nd edn. Wiley, New York, p 242
Nicholson RS, Shain I (1964) Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal Chem 36:706
Retter U, Lohse H (2012) In: Scholz F (ed) Electrochemical methods, 2nd edn. Heidelberg, Springer
Acknowledgments
This study was initially supported by the Natural Sciences and Engineering Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oldham, K.B. Ultimate cyclic voltammetry: an analytical examination of the reversible case. J Solid State Electrochem 17, 2749–2756 (2013). https://doi.org/10.1007/s10008-013-2176-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2176-2