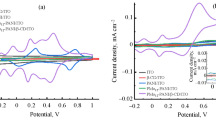
Abstract
Poly(vinylferrocenium) perchlorate–polyaniline (PVF+–PANI) composite film was synthesized electrochemically on Pt electrode in a methylene chloride solution containing a mixture of poly(vinylferrocene) (PVF) polymer and aniline monomer. PVF+ polymer in the composite film was used as an electron transfer mediator. The composite coating showed significant electrochemical activity towards hydroquinone (HQ) at pH 4, with high sensitivity and a wide linearity range. The interaction of HQ with PVF+ and PANI homopolymer films was investigated electrochemically and spectroscopically. HQ molecules are accumulated on the electrode surface due to trapping by both polymers in the composite film and then oxidized catalytically by PANI. The most significant contribution of PVF+ polymer is that it facilitates electron transfer in the composite film. The linear response range was found to be between 1.60 × 10−4 mM and 115 mM (R 2 = 0.999) at 0.45 V vs saturated calomel electrode. The limit of detection (LOD) was 4.94 × 10−5 mM.












Similar content being viewed by others
References
Rajesh TW, Kaneto K (2004) Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly(N-3-aminopropyl pyrrole-co-pyrrole) film. Sensor Actuat B-Chem 102(2):271–277
Scobbie E, Groves JA (1999) Determination of hydroquinone in air by high performance liquid chromatography. Ann Occup Hyg 43(2):131–141
Xie TY, Liu QW, Shi YR, Liu QY (2006) Simultaneous determination of positional isomers of benzenediols by capillary zone electrophoresis with square wave amperometric detection. J Chromatogr A 1109(2):317–321
Lin CH, Sheu JY, Wu HL, Huang YL (2005) Determination of hydroquinone in cosmetic emulsion using microdialysis sampling coupled with high-performance liquid chromatography. J Pharmaceut Biomed 38(3):414–419
Ziyatdinova G, Gainetdinova A, Morozov M, Budnikov H, Grazhulene S, Red’kin A (2011) Voltammetric detection of synthetic water-soluble phenolic antioxidants using carbon nanotube based electrodes. J Solid State Electrochem. doi:10.1007/s10008-011-1295-x
Skladal P, Morozova NO, Reshetilov AN (2002) Amperometric biosensors for detection of phenol using chemically modified electrodes containing immobilized bacteria. Biosens Bioelectron 17(10):867–873
Erhan E, Korkut S, Keskinler B (2008) An amperometric biosensor based on multiwalled carbon nanotube-poly(pyrrole)-horseradish peroxidase nanobiocomposite film for determination of phenol derivatives. Talanta 76(5):1147–1152
Zeng GM, Zhang Y, Tang L, Huang DL, Jiang XY, Chen YN (2007) A hydroquinone biosensor using modified core-shell magnetic nanoparticles supported on carbon paste electrode. Biosens Bioelectron 22(9–10):2121–2126
Russell IM, Burton SG (1999) Development and demonstration of an immobilised-polyphenol oxidase bioprobe for the detection of phenolic pollutants in water. Anal Chim Acta 389(1–3):161–170
Kochana J, Nowak P, Jarosz-Wilkolazka A, Bieron M (2008) Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem J 89(2):171–174
Yano J, Ogura K, Kitani A, Sasaki K (1992) The kinetic difference between hydroquinone and Fe2+ in the electrochemical response of a polyaniline-film-coated electrode. Synthetic Met 52(1):21–31
Inzelt G (2011) Rise and rise of conducting polymers. J Solid State Electrochem. doi:10.1007/s10008-011-1338-3
Pekmez N, Pekmez K, Yildiz A (1994) Electrochemical behavior of polyaniline films in acetonitrile. J Electroanal Chem 370(1–2):223–229
Yano J, Kokura M, Ogura K (1994) Electrocatalytic behavior of a poly(N-methylaniline) filmed electrode to hydroquinone. J Appl Electrochem 24(11):1164–1169
Mu SL (2003) The electrocatalytic oxidation of gallic acid on polyaniline film synthesized in the presence of ferrocene phosphonic acid. Synthetic Met 139(2):287–294
Peerce PJ, Bard AJ (1980) Polymer-films on electrodes. 2. Film structure and mechanism of electron-transfer with electrodeposited poly(vinylferrocene). J Electroanal Chem 112(1):97–115
Gulce H, Ozyoruk H, Yildiz A (1995) Electrochemical response of poly(vinylferrocenium)-coated Pt electrodes to some anions in aqueous-media. Electroanal 7(2):178–183
Gulce H, Gulce A, Kavanoz M, Coskun H, Yildiz A (2002) A new amperometric enzyme electrode for alcohol determination. Biosens Bioelectron 17(6–7):517–521
Inzelt G, Bacskai J (1992) Electrochemical quartz crystal microbalance study of the swelling of poly(vinylferrocene) films. Electrochim Acta 37(4):647–654
Smith TW, Kuder JE, Wychick D (1976) Voltammetric behavior of poly(vinylferrocene). J Polym Sci Pol Chem 14(10):2433–2448
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York, p 5
Vianello R, Kovacevic B, Ambrozic G, Mavri J, Maksic ZB (2004) Hydrogen bonding in complex of serine with histidine: computational and spectroscopic study of model compounds. Chem Phys Lett 400:117–121
Zhang J, Wang L, Zhang H, Wang R (2003) Ab initio study for the effect of hydrogen bonds in the intermolecular interaction between high-spin molecules. Synthetic Met 137:1349–1350
Stejskal J, Blinova NV, Trchova M, Prokes J, Omastova M (2007) Polyaniline and polypyrrole: a comparative study of the preparation. Eur Polym J 43(6):2331–2341
Gok A, Omastova M, Prokes J (2007) Synthesis and characterization of red mud/polyaniline composites: electrical properties and thermal stability. Eur Polym J 43(6):2471–2480
Yang JP, Ding Y, Chen G, Li C (2007) Synthesis of conducting polyaniline using novel anionic Gemini surfactant as micellar stabilizer. Eur Polym J 43(8):3337–3343
Chao D, Chen J, Lu X, Liang Chen L, Zhang W, Wei Y (2005) SEM study of the morphology of high molecular weight polyaniline. Synthetic Met 150:47–51
Sulu Hasik M, Paluszkiewicz C, Bielanska E (2005) Reactions of polyaniline with transition metal ions in nonaqueous solutions. J Mol Struct 744–747:677–683
Pandey PC, Singh G (2001) Tetraphenylborate doped polyaniline based novel pH sensor and solid-state urea biosensor. Talanta 55:773–782
Can M, Uzun S, Pekmez NO (2009) Chemical polymerization of aniline using periodic acid in acetonitrile. Synthetic Met 159:1486–1490
Huang WS, Humphrey BD, Macdiarmid AG (1986) Polyaniline, a novel conducting polymer—morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J Chem Soc Farad T 1 82:2385
Zagorska M, Pron A, Leftrant S (1997) In: Nalwa HS (ed) Handbook of organic conductive molecules and polymers, vol 3. Wiley, New York, p 183
Lee CW, Jin SH, Yoon KS, Jeong HM, Chi KW (2009) Efficient oxidation of hydroquinone and alcohols by tailor-made solid polyaniline catalyst. Tetrahedron Lett 50(5):559–561
Tang JS, Osteryoung RA (1991) Formation and electrochemistry of polyaniline in ambient-temperature molten-salts. Synthetic Met 45(1):1–13
Pekmez NO, Can M, Yildiz A (2007) Spectroscopic and electrochemical observation of hydrogen-bonded ımidazole and 2-aminoimidazole clusters. Acta Chi Slov 5:131–139
Bugg TDH, Lin G (2001) Solving the riddle of the intradiol and extradiol catechol dioxygenases: how do enzymes control hydroperoxide rearrangements? Chem Commun 11:941–952
Wei WZ, Kong B, Yin TJ, Liu XY (2007) Voltammetric determination of hydroquinone using beta-cyclodextrin/poly(N-acetylaniline)/carbon nanotube composite modified glassy carbon electrode. Anal Lett 40(11):2141–2150
Ghanem MA (2007) Electrocatalytic activity and simultaneous determination of catechol and hydroquinone at mesoporous platinum electrode. Electrochem Commun 9(10):2501–2506
Zeng GM, Zhang Y, Tang L, Huang DL, Jiang XY, Chen YN (2007) A hydroquinone biosensor using modified core-shell magnetic nanoparticles supported on carbon paste electrode. Biosens Bioelectron 22(9–10):2121–2126
Wang L, Huang PF, Bai JY, Wang HJ, Zhang LY, Zhao YQ (2007) Covalent modification of a glassy carbon electrode with penicillamine for simultaneous determination of hydroquinone and catechol. Microchim Acta 158(1–2):151–157
Fang B, Sun JG, Wang GF, Yu Y, Jiao SF (2007) Preparation and application of La(OH)(3) nanoparticles self-assembled film modified electrode. Anal Lett 40(4):705–714
Long GL, Winefordner JD (1983) Limit of detection. Anal Chem 55(7):712A–724A
Acknowledgments
Support of this work by Scientific Research Department of Hacettepe University (project no. 0801601011) is greatly appreciated. The authors kindly thank Dr. Muzaffer Can for theoretical calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavanoz, M., Pekmez, N.Ö. Poly(vinylferrocenium) perchlorate–polyaniline composite film-coated electrode for amperometric determination of hydroquinone. J Solid State Electrochem 16, 1175–1186 (2012). https://doi.org/10.1007/s10008-011-1505-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1505-6