Abstract
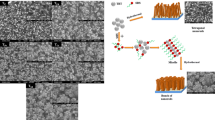
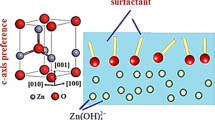
In this paper, we report on the nickel oxide (NiO) thin films potentiostatically electrodeposited onto indium-doped tin oxide-coated glass substrates by using two types of organic surfactants: (1) non-ionic: polyethylene glycol (PEG), polyvinylpyrrolidone (PVP) and (2) anionic: sodium dodecyl sulfate (SDS). An aqueous solution containing nickel sulfate precursor and potassium hydroxide buffer was used to grow the samples. The effect of organic surfactants on its structural, morphological, wettability, optical, electrochromic, and in situ colorimetry were studied using X-ray diffraction, scanning electron microscopy, contact angle, FT-IR spectroscopy, optical transmittance, cyclic voltammetry, and CIE system of colorimetry. X-ray diffraction patterns show that the films are polycrystalline, consisting of NiO cubic phase. A nanoporous structure with pore diameter of about 150–200 nm was observed for pure NiO. The films deposited with the aid of organic surfactants exhibits various surface morphological feature. PVP-mediated NiO thin film shows noodle-like morphology with well-defined surface area whereas, an ordered pore structure composed of channels of uniform diameter of about 60–80 nm was observed for PEG. A compact and smooth surface with nanoporous structure stem from SDS helps for improved electrochromic performance compared with that of NiO deposits from surfactant-free solution. Wetting behavior shows, transformation from hydrophilic to superhydrophilic nature of NiO thin films deposited with organic surfactant, which helps for much more paths for electrolyte access. The surfactant-mediated NiO produce high color/bleach transmittance difference up to 57% at 630 nm. On oxidation of NiO/SDS, the CIELAB 1931 2° color space coordinates show the transition from colorless to the deep brown state (L* = 84.41, a* = −0.33, b* = 4.41, and L* = 43.78, a* = 7.15, b* = 13.69), with steady decrease in relative luminance. The highest coloration efficiency of 54 cm2 C−1 with an excellent reversibility of 97% was observed for NiO/SDS thin films.












Similar content being viewed by others
References
Granqvist CG (1995) Handbook of inorganic electrochromic materials. Elsevier, Amsterdam, pp 162–165
Yu PC, Nazri G, Lampert CM (1987) Sol Energy Mater 19:1
Hotovy I, Huran J, Spiess L, Hascik S, Rehacek V (1999) Sens Actuat B 57:147–152
Nattestad A, Ferguson M, Kerr R, Cheng Yi-B, Bach U (2008) Nanotechnology 19(295304):3852–3854
Ghosh M, Biswas K, Sundaresan A, Rao CNR (2006) J Mater Chem 16:106–111
Wei B, Yamamoto S, Ichikawa M, Li C, Takeshi F, Taniguchi Y (2007) Semicond Sci Technol 22:788–792
Sreethawong T, Suzuki Y, Yoshikawa S (2005) Int J Hydrogen Energy 30:1053–1062
Gomes A, da Silva Pereira MI (2006) Electrochim Acta 51:1342–1350
Kavinchan J, Thongtem T, Thongtem S (2010) Mater Lett 61:2388–2391
Zhu Z, Wei N, Liu H, Hea Z (2010) Advanced powder technology, In Press, Corrected Proof, Available online 1 July (doi:10.1016/j.apt.2010.06.008)
Cavalcante LS, Sczancoski JC, Tranquilin RL, Varela JA, Longo E, Orlandi MO (2009) Particuology 7:353–362
Thongtem T, Pilapong C, Kavinchan J, Phuruangrat A, Thongtem S (2010) J Alloy Comp 500:195–199
Phuruangrat A, Thongtem T, Thongtem S (2009) J Alloy Compd 481:568–572
Thongtem T, Jattukul S, Phuruangrat A, Thongtem S (2010) J Alloy Compd 491:654–665
Moura AP, Cavalcante LS, Sczancoski JC, Stroppa DG, Paris EC, Ramirez AJ, Varela JA, Longo E (2010) Adv Powder Technol 21:197–202
Inamdar AI, Mujawar SH, Ganesan V, Patil PS (2008) Nanotechnology 19:325706
Retter U, Tchachnikova M (2003) J Electroanal Chem 550:201–208
Opydo J (1992) Talanta 39:229–234
Lai WH, Shieh J, Teoh LG, Hung IM, Liao CS, Hon MH (2005) J Alloy Compd 396:295–301
Tan Y, Srinivasan S, Choi K-S (2005) J Am Chem Soc 127:3596–3604
Lee J, Hwang DK, Choi JM, Lee K, Kim JH, Im S (2005) Appl Phys Lett 87:023504
Nelson PA, Elliott JM, Attard GS, Owen JR (2002) J New Mater Electrochem Syst 5:63–65
Xia XH, Tu JP, Zhang J, Wang XL, Zhang WK, Huang H (2008) Electrochim Acta 53:5721–5724
Purushothaman KK, Muralidharan G (2008) J Sol Gel Sci Technol 46:90–94
Kamal H, Elmaghraby EK, Ali SA, Abdel-Hady K (2004) J Cryst Growth 262:424–434
Wang X, Song J, Gao L, Jin Ji, Zheng H, Zhang Z (2005) Nanotechnology 16:37
Uplane MM, Mujawar SH, Inamdar AI, Shinde PS, Sonavane AC, Patil PS (2007) Appl Surf Sci 251:9365–9371
Maruyama T, Arai S (1993) Sol Energy Mater Sol Cells 30:257–262
Penin N, Rougier A, Laffont L, Poizot P, Tarascon JM (2006) Sol Energy Mater Sol Cells 90:422–433
Nagai J (1993) Sol Energy Mater Sol Cells 31:291–299
Ahn KS, Nah YC, Sung YE (2003) Solid State Ionics 165:155–160
Avendan E, Azens A, Isidorsson J, Karmhag R, Niklasson GA, Granqvist CG (2003) Solid State Ionics 165:169–173
Wu MS, Yang CH (2007) Appl Phys Lett 91:033109
Huo QS, Margolese DI, Ciesla U, Demuth DG, Feng PY, Gier TE, Sieger P, Firouzi A, Chmelka BF, Schuth F, Stucky GD (1994) Chem Mater 6:1176–1181
Kadam LD, Patil PS (2001) Sol Energy Mater Sol Cells 69:361–369
Korosec RC, Ogorevc JS, Draskovic P, Drazic G, Bukovec P (2008) Thin Solid Films 516:8264–8271
Matar OK, Craster RV (2009) Soft Matter 5:801–808
Peng X, Chen A (2005) Appl Phys A 80:473–476
Dalavi DS, Suryavanshi MJ, Patil DS, Mali SS, Mohalkar AV, Kalagi SS, Vanalkar SA, Kang SR, Kim JH, Patil PS (2011) Appl Surf Sci. 257:2647–2656
Ezema FI, Ekwealor ABC, Osuji RU (2008) Superficies y Vacío 21(1):6–10
Sonavane AC, Inamdar AI, Shinde PS, Deshmukh HP, Patil RS, Patil PS (2010) J Alloy Compd 489:667–673
Lou X, Zhao X, Feng J, Zhou X (2009) Prog Org Coat 64:300–307
Kalagi SS, Dalavi DS, Pawar RC, Tarwal NL, Mali SS, Patil PS (2010) J Alloy Compd 493:335–339
CIE, Colorimetry (Official Recommendations of the International Commission on illumination) (1971) CIE Publication No15 Paris
Song HK, Lee EJ, Oh SM (2005) Chem Mater 17:2232–2233
Mortimer RJ, Reynolds JR (2005) J Mater Chem 15:2226–2233
Fei J, Lim KG, Palmore GTR (2008) Chem Mater 20(12):3832–3839
Acknowledgment
One of the authors, Mr. D. S. Dalavi, is thankful to University Grants Commission (UGC) for the award of Rajiv Gandhi Junior Research Fellowship and UGC-New Delhi for the financial support though UGC-New Delhi Project F.No.211/2008 (SR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
(AVI 1875 kb)
Rights and permissions
About this article
Cite this article
Dalavi, D.S., Suryavanshi, M.J., Mali, S.S. et al. Efficient maximization of coloration by modification in morphology of electrodeposited NiO thin films prepared with different surfactants. J Solid State Electrochem 16, 253–263 (2012). https://doi.org/10.1007/s10008-011-1314-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1314-y