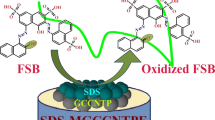
Abstract
Electrochemical behavior of bisphenol A (BPA) at glassy carbon electrode-modified with layered double hydroxide (LDH) and anionic surfactant (sodium dodecyl sulfate) is investigated by electrochemical techniques. Compared with the bare electrode and LDH-modified electrode, the oxidation peak potential of BPA shifted negatively and the peak current increased significantly due to the enhanced accumulation of BPA via electrostatic interaction with LDH at the hydrophobic electrode surface. Some determination conditions such as LDH loading, pH, scan rate, accumulation potential, and accumulation time on the oxidation of BPA were optimized. And some kinetic parameters were investigated. Under the optimized conditions, the oxidation current was proportional to BPA concentration in the range of 8 × 10−9 to 2.808 × 10−6 M with the detection limit of 2.0 × 10−9 M by amperometry. The fabricated electrode showed good reproducibility, stability, and anti-interference. The proposed method was successfully applied to determine BPA in water samples, and the results were satisfactory.



Similar content being viewed by others
References
Kloas W, Lutz I, Einspanier R (1999) Sci Total Environ 225:59–68
Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N (1997) Endocrinology 138:1780–1786
Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D (1993) Endocrinology 132:2279–2286
Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M (2001) Environ Sci Technol 35:2917–2925
Hiroi H, Tsutsumi O, Momoeda M, Takai Y, Osuga Y, Taketani Y (1999) Endocrine Journal 46:773–778
Safe SH (2000) Environ Health Perspect 108:487–493
Staples C, Dome P, Klecka G, Oblock S, Harris L (1998) Chemosphere 36:2149–2173
Goodson A, Robin H, Summerfield W, Cooper I (2004) Food Addi Contam 21:1015–1026
Lopez-Cervantes J, Paseiro-Losada P (2003) Food Addi Contam 20:596–606
Wen Y, Zhou B, Xu Y, Jin S, Feng Y (2006) J Chromatogr A 1133:21–28
Shin H, Park C, Park S, Pyo H (2001) J Chromatogr A 912:119–125
Sáchez-Acevedo ZC, Riu J, Rius FX (2009) Biosens Bioelectron 24:2842–2846
Cavani F, Trifiro F, Vaccari A (1991) Catal Today 11:173–301
Pavan PC, Crepaldi EL, Valim JB (2000) J Colloid Interface Sci 229:346–352
Fernández L, Borrás C, Carrero H (2006) Electrochim Acta 52:872–884
Hernandez M, Fernandez L, Borras C, Mostany J, Carrero H (2007) Anal Chim Acta 597:245–256
Klumpp E, Contreras-Ortega C, Klahre P, Tino FJ, Yapar S, Portillo C, Stegen S, Queirolo F, Schwuger MJ (2003) Colloids Surf A 230:111–116
Ulibarri MA, Pavlovic I, Barriga C, Hermosín MC, Cornejo J (2001) Appl Clay Sci 18:17–27
Fernández L, Carrero H (2005) Electrochim Acta 50:1233–1240
Scavetta E, Ballarin B, Berrettoni M, Carpani I, Giorgetti M, Tonelli D (2006) Electrochim Acta 51:2129–2134
Zhang L, Zhang Q, Lu X, Li J (2007) Biosens Bioelectron 23:102–106
Yin H, Cui L, Ai S, Fan H, Zhu L (2009) Electrochim Acta 55:603–610
Kannan S (2004) J Mater Sci 39:6591–6596
Kuramitz H, Nakata Y, Kawasaki M, Tanaka S (2001) Chemosphere 45:37–43
Tanaka S, Nakata Y, Kimura T, Kawasaki M, Kuramitz H (2002) J Appl Electrochem 32:197–201
Yin H, Zhou Y, Ai S (2009) J Electroanal Chem 626:80–88
Murugananthan M, Yoshihara S, Rakuma T, Shirakashi T (2008) J Hazard Mater 154:213–220
Ngundi M, Sadik O, Yamaguchi T, Suye S (2003) Electrochem Commun 5:61–67
Yin H, Zhou Y, Xu J, Ai S, Cui L, Zhu L (2010) Anal Chim Acta 659:144–150
Yin H, Zhou Y, Ai S, Chen Q, Zhu X, Liu X, Zhu L (2009) J Hazard Mater 174:236–243
Ballesteros-Gómez A, Rubio S, Pérez-Bendito D (2009) J Chromatogr A 1216:449–469
Luckza T (2008) Electrochim Acta 53:5725–5731
Laviron E (1974) J Electroanal Chem 52:355–393
Dempsey E, Diamond D, Collier A (2004) Biosens Bioelectron 20:367–377
Mita D, Attanasio A, Arduini F, Diano N, Grano V, Bencivenga U, Rossi S, Amine A, Moscone D (2007) Biosens Bioelectron 23:60–65
Notsu H, Tatsuma T, Fujishima A (2002) J Electroanal Chem 523:86–92
Andreescu S, Sadik O (2004) Anal Chem 76:552–560
Wang F, Yang J, Wu K (2009) Anal Chim Acta 638:23–28
Huang W (2005) Bull Korean Chem Soc 26:1560–1564
Chauke V, Matemadombo F, Nyokong T (2010) J Hazard Mater 178:180–186
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 20775044) and the Natural Science Foundation of Shandong province, China (Y2006B20).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yin, H., Zhou, Y., Cui, L. et al. Electrochemical oxidation behavior of bisphenol A at surfactant/layered double hydroxide modified glassy carbon electrode and its determination. J Solid State Electrochem 15, 167–173 (2011). https://doi.org/10.1007/s10008-010-1089-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1089-6