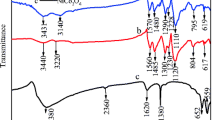
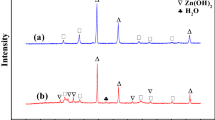
Abstract
An organic–inorganic poly(3,4-ethylenedioxythiophene) (PEDOT)/RuO2·xH2O nanocomposite (approximately 1 wt.% RuO2) has been successfully prepared for the first time under microwave irradiation within 5 min with power 900 W via in situ chemical polymerization. The morphology and structure of the resultant material is characterized by transmission electron microscope and Fourier transform infrared. Moreover, the electrochemical properties of the synthesized nanocomposite can be controlled by adjusting the annealing temperature, which is definitely illustrated by cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectra. Electrochemical data have shown that the PEDOT/RuO2·xH2O nanocomposite annealed at 150 °C possesses the most favorable charge/discharge ability with a specific capacitance of 153.3 F g−1 at a current density of 150 mA g−1 and the high efficient utilization of PEDOT at various current densities. Furthermore, such composite has a less capacitance degradation of 23.8% after 1,000 continuous cycles. The improved electrochemical performance are mainly attributed to the large electroactive surface of nanocomposite and the existence of amorphous RuO2·xH2O particles as well as a synergistic effect of the polymer PEDOT and annealed RuO2·xH2O. Thus, the PEDOT/RuO2·xH2O nanocomposite annealed at 150 °C can act as a promising electroactive material for supercapacitor application.










Similar content being viewed by others
Reference
Colvin VL, Schlamp MC, Alivisatos AP (1994) Nature 370:354. doi:10.1038/370354a0
Arbizzani C, Mastragostino M, Meneghello L, Paraventi R (1996) Adv Mater 8:331. doi:10.1002/adma.19960080409
Baraton MI, Merhara L, Wang J, Gonsalves KE (1998) Nanotechnology 9:356. doi:10.1088/0957-4484/9/4/010
Rajesh B, Thambi KR, Bonard JM, Xanthapolous N, Mathieu HJ, Viswanathan B (2002) Electrochem Solid-State Lett 5:E71. doi:10.1149/1.1518610
Mattes BR, Knobbe ET, Fuqua PD, Nishid F, Chang EW, Pierce BM, Dunn B, Karner RB (1991) Synth Met 43:3183. doi:10.1016/0379-6779(91)91262-9
Rios EC, Rosario AV, Mello RM, Micaroni L (2007) J Power Sources 163:1137. doi:10.1016/j.jpowsour.2006.09.056
Zhang L, Wang M (2003) J Phys Chem B 107:6748. doi:10.1021/jp034130g
Zhang X, Lee JS, Lee GS, Cha DK, Moon JK, Yang DJ, Manohar SK (2006) Macromolecules 39:470. doi:10.1021/ma051975c
Gustafsson JC, Liedberg B, Inganas O (1994) Solid State Ion 69:145. doi:10.1016/0167-2738(94)90403-0
Chiu WW, Sejdic JT, Cooney RP, Bowmaker GA (2005) Synth Met 155:80. doi:10.1016/j.synthmet.2005.06.012
Muruganand AV, Viswanath AK (2006) J Appl Phys 100:074319. doi:10.1063/1.2356788
Liu R, Lee SB (2008) J Am Chem Soc 130:2942. doi:10.1021/ja7112382
Wang YG, Zhang XG (2004) Electrochim Acta 49:1957. doi:10.1016/j.electacta.2003.12.023
Panic V, Vidakovic T, Gojkovic S, Dekanski A, Milonjic S, Nikolic B (2003) Electrochim Acta 48:3805. doi:10.1016/S0013-4686(03)00514-0
Trasatti S, Kurzweil P (1994) Platin Met Rev 46:38
Fang QL, Evans DA, Roberson SL, Zheng JP (2001) J Electrochem Soc 148:A833. doi:10.1149/1.1379739
Hong JI, Yeo IH, Paika WK (2001) J Electrochem Soc 148:A156. doi:10.1149/1.1342166
Huang LM, Wen TC, Gopalan A (2006) Electrochim Acta 51:3469. doi:10.1016/j.electacta.2005.09.049
Han DX, Yang GF, Niu L, Ivaska A (2007) J Electroanal Chem 602:24. doi:10.1016/j.jelechem.2006.11.027
Wang JC, Yu XF, Li YX, Liu Q (2007) J Phys Chem C 111:18073. doi:10.1021/jp0749468
Hu CC, Liu MJ, Chang KH (2007) J Power Sources 163:1126. doi:10.1016/j.jpowsour.2006.09.060
Chen WC, Hu CC (2004) J Power Sources 125:292. doi:10.1016/j.jpowsour.2003.08.001
Liang YY, Li HL, Zhang XG (2007) J Power Sources 173:599. doi:10.1016/j.jpowsour.2007.08.010
Acknowledgements
This work was supported by National Basic Research Program of China (973 Program no. 2007CB209703) and National Natural Science Foundation of China (nos. 20633040, 20873064), Graduate Innovation Plan of Jiangsu Province (CX07B-089Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Yuan, C., Gao, B. et al. Microwave-assisted synthesis of organic–inorganic poly(3,4-ethylenedioxythiophene)/RuO2·xH2O nanocomposite for supercapacitor. J Solid State Electrochem 13, 1925–1933 (2009). https://doi.org/10.1007/s10008-008-0777-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0777-y