Abstract
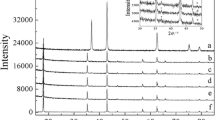
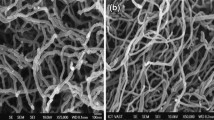
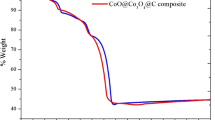
Olivine-type LiFePO4 is one of the most promising cathode materials for lithium-ion batteries, but its poor conductivity and low lithium-ion diffusion limit its practical application. The electronic conductivity of LiFePO4 can be improved by carbon coating and metal doping. A small amount of La-ion was added via ball milling by a solid-state reaction method. The samples were characterized by X-ray diffractometer (XRD), scanning electron microscopy (SEM)/mapping, differential scanning calorimetry (DSC), transmission electron microscopy (TEM)/energy dispersive X-ray spectroscopy (EDS), and total organic carbon (TOC). Their electrochemical properties were investigated by cyclic voltammetry, four-point probe conductivity measurements, and galvanostatic charge and discharge tests. The results indicate that these La-ion dopants do not affect the structure of the material but considerably improve its rate capacity performance and cyclic stability. Among the materials, the LiFe0.99La0.01PO4/C composite presents the best electrochemical behavior, with a discharge capacity of 156 mAh g−1 between 2.8 and 4.0 V at a 0.2 C-rate compared to 104 mAh g−1 for undoped LiFePO4. Its capacity retention is 80% after 497 cycles for LiFe0.99La0.01PO4/C samples. Such a significant improvement in electrochemical performance should be partly related to the enhanced electronic conductivities (from 5.88 × 10−6 to 2.82 × 10−3 S cm−1) and probably the mobility of Li+ ion in the doped samples. The LiFe0.99La0.01PO4/C composite developed here could be used as a cathode material for lithium-ion batteries.











Similar content being viewed by others
References
Ozawa K (1994) Solid State Ion 69:212
Whittingham MS (2000) Solid State Ion 134:169
Armstrong AR, Bruce PG (1996) Nature 381:499
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188
Zane D, Carewska M, Scaccia S, Cardellini F, Prosini PP (2004) Electrochimica Acta 49:4259
Konstantinov K, Bewlay S, Wang GX, Lindsay M, Wang JZ, Liu HK, Dou SX, Ahn J-H (2004) Electrochim Acta 50:421
Gabersceka M, Dominkoa R, Belea M, Remskarb M, Hanzelb D, Jamnika J (2005) Solid State Ion 176:1801
Yang M-R, Teng T-H, Wu S-H (2006) J Power Sources 159:307
Nakamura T, Miwa Y, Tabuchi M, Yamada Y (2006) J Electrochem Soc 153:A1108
Park KS, Son JT, Chung HT, Kim SJ, Lee CH, Kang KT, Kim HG (2004) Solid State Comm 129:311
Gabrisch H, Wilcox JD, Doeff MM (2006) Electrochem Solid-State Lett 9:A360
Barker J, Saidi MY, Swoyer JL (2003) Electrochem Solid-State Lett 6(3):A53–A55
Salah AA, Mauger A, Julien CM, Gendron F (2006) Materials Science and Engineering B 129:232
Dong Q, Liu S, Zheng M, Zhan Y, Sun S, Lin Z (2006) Abstract 111, 209th ECS Meeting, May 7–12, Denver, Colorado
Eftekhari A (2004) J Electrochem Soc 151:A1456
Dominko R, Gaberscek M, Drofenik J, Bele M, Pejovnik S, Jamnik J (2003) J Power Sources 119–121:770
Zhang SS, Allen JL, Xu K, Jow TR (2005) J Power Sources 147:234–240
Xu Y, Lu Y, Yan L, Yang Z, Yang R (2006) J Power Sources 160:570
Herle PS, Ellis B, Coombs N, Nazar LF (2004) Nature mater 3:147
Chung SY, Blocking JT, Chiang YM (2002) Nature Mater 2:123
Ni JF, Zhou HH, Chen JT, Zhang XX (2005) Mater Lett 59:2361
Abbate M, Lala SM, Montoro LA, Rosolen JM (2005) Electrochem Solid-State Lett 8:A288
Wang GX, Bewlay S, Yao J, Ahn JH, Dou SX, Liu HK (2004) Electrochem Solid-State Lett 7:A503
Chung SY, Bloking J, Chiang YM (2002) Nat Mater 1:123
Herle PS, Ellis B, Coombs N, Nazar LF (2004) Nat Mater 3:147
Cullity BD, Stock SR, Elements of X-ray Diffraction (2001) Prentice Hall Publishers, New Jersey, USA, 3rd, Ch 5.2
Chen Z, Dahn JR (2002) J Electrochem 8:450
Zhang Z, Fouchard D, Rea JR (1998) J Power Sources 70:16
Ravet N, Abouimrane A, Amand M (2003) Nat Matters 2:702
Burba CM, Frech R (2004) J Electrochem Soc 151:A1032
Doeff MM, Hu Y, McLarnon F, Kostecki R (2003) Electrochem Solid-State Lett 6:A207
Hu Y, Doeff MM, Kostecki R, Finones R (2004) J Electrochem Soc 151:A1279
Doeff MM, Hu Y, McLarnon F, Kostecki R (2003) Electrochem. Solid State Lett 6:A207
Fey GTK, Lu TL (2008) J Power Sources 178:807
Acknowledgement
The authors thank Prof. W. H. Li (Department of Physics, National Central University) for his valuable suggestions and the use of Raman spectrometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contribution to ICMAT 2007, Symposium K: Nanostructural and bulk materials for electrochemical power sources, July 1–6, 2007, Singapore
Rights and permissions
About this article
Cite this article
Cho, YD., Fey, G.TK. & Kao, HM. Physical and electrochemical properties of La-doped LiFePO4/C composites as cathode materials for lithium-ion batteries. J Solid State Electrochem 12, 815–823 (2008). https://doi.org/10.1007/s10008-007-0498-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-007-0498-7