Abstract
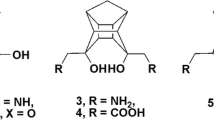
The enzyme-binding mode of a series of interleukin-1β converting enzyme (ICE) inhibitors has been analysed on the basis of the crystal structure of the complex between hICE and tetrapeptide aldehyde. The conformation of these ligands were explored by performing molecular dynamics simulations at 100 ps. The conformation adopted by these inhibitors was very similar to and could be superimposable onto the regions of crystal structure. The active and the low energy conformers were docked either by grid or manually into the binding site. The analysis of the resulting model indicated that O-benzylacyl group of aspartyl hemiacetals interact with Cys285 and the large substituents: semicarbazone, 2,6-bis(trifluoromethyl) benzoate, other leaving groups of (acyloxy)methyl and α-((2,6-dichlorobenzoyl)oxy)methyl ketone series of P1 site protrude from the surface of Cys285 and interact with Val338, which is located below the binding pocket. The hydrogen bonding interaction between -NH of semicarbazone and Cys285 seems to have significant role. The total potential energy including intermolecular interaction energy, consisting of van der Waals and electrostatic energies were calculated. The resulting model is qualitatively consistent with the reported experimental data and can be useful for the design of more potent inhibitors of ICE.
Similar content being viewed by others
Author information
Authors and Affiliations
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Hariprasad, V., Kulkarni, V. Docking of a Series of Peptide-Based Interleukin-1β Converting Enzyme Inhibitors with Aspartyl Hemiacetals, α-((2,6-Dichlorobenzoyl)oxy)methyl and (Acyloxy)methyl Ketone Moieties. J Mol Model 3, 443–454 (1997). https://doi.org/10.1007/s008940050062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s008940050062