Abstract
Context
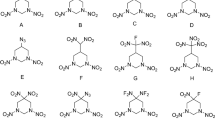
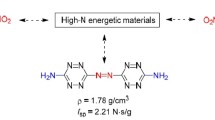
Explosive properties of novel potential high energy density materials of a series of 1,2-diazete-based molecules with trinitromethyl functional group were investigated computationally. All the sixty seven molecules were optimised to obtain their molecular geometries and electronic structures. Electrostatic potential analysis was also carried out in the determination of different parameters. The calculations indicate that the majority of the compounds have high positive heat of formations, high density and good detonation performance greater than that of traditional energetic materials like RDX and HMX. They are also having comparable values of impact sensitivity. These features promise their potential to be used as energetic materials for future applications. Most of the designed molecules are having high positive oxygen balance values so that the study of these molecules can also be extended as potential candidates for oxidisers in solid propellants.
Methods
Optimisation and vibrational frequency analysis of the studied molecules were done with density functional theory using B3LYP/aug-cc-pVDZ as the basis set to zero imaginary frequencies using Gaussian 09. Electrostatic potential analysis were carried using the Multiwfn program.
Graphical Abstract









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Khan RU, Zhu S, Zhu W (2019) DFT studies on nitrogen-rich pyrazino [2, 3-e][1, 2, 3, 4] tetrazine-based high-energy density compounds. J Mol Model 25:283
Li B, Zhou M, Peng J, Li L, Guo Y (2019) Theoretical calculations about nitro-substituted pyridine as high-energy-density compounds (HEDCs). J Mol Model 25:23
Qiu L, Gong X, Zheng J, Xiao H (2009) Theoretical studies on polynitro-1, 3-bishomopentaprismanes as potential high energy density compounds. J Hazard Mater 166:931–938
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP (2008) Advances in science and technology of modern energetic materials: an overview. J Hazard Mater 151:289–305
Gupta S, Singh HJ (2020) Computational studies on nitro derivatives of BN indole as high energetic material. J Mol Model 26:1–10
Zhao XX, Li SH, Wang Y, Li YC, Zhao FQ, Pang SP (2016) Design and synthesis of energetic materials towards high density and positive oxygen balance by N-dinitromethyl functionalization of nitroazoles. J Mater Chem A 4:5495–5504
Klapötke TM, Leroux M, Schmid PC, Stierstorfer J (2016) Energetic Materials Based on 5, 5′-Diamino-4, 4′-dinitramino-3, 3′-bi-1, 2, 4-triazole. Chem Asian J 11:844–851
Wang B, Ren F, Shi W (2015) A theoretical investigation into the strength of N-NO2 bonds, ring strain and electrostatic potential upon formation of intermolecular H-bonds between HF and the nitro group in nitrogen heterocyclic rings CnH2nN-NO2(n = 2–5), RDX and HMX. J Mol Model 21:1–11
Zhang J, Mitchell LA, Parrish DA, Shreeve JM (2015) Enforced layer-by-layer stacking of energetic salts towards high-performance insensitive energetic materials. J Am Chem Soc 137:10532–10535
Chen B, Lu H, Chen J, Chen Z, Yin SF, Peng L, Qiu R (2023) Recent progress on nitrogen-rich energetic materials based on tetrazole skeleton. Top CurrChem 381:25
Yin Y, Yao E, Xiao L, Bai J, Ren Y, Ma H, Zhao F, Shen W (2023) Bis (5-nitroimino-1, 2, 4-triazole-3-yl) methane-based energetic salts: synthesis, crystal structure, thermal behavior and catalytic activity. J Mol Struct 1273:134195
Lang Q, Sun Q, Xu Y, Wang P, Lin Q, Lu M (2021) From mono-rings to bridged bi-rings to caged bi-rings: a promising design strategy for all-nitrogen high-energy-density materials N10 and N12. New J Chem 45:6379–6385
Liu Y, Yu T, Lai W, Ma Y, Ge Z, Yang FL, Liang PY, Long Y, Zhou PP, Yang Z (2021) High-energetic and low-sensitive 1, 3, 5-triamino 2, 4, 6-trinitrobenzene (TATB) crystal: first principles investigation and Hirshfeld surface analysis. New J Chem 45:6136–6143
Manna MS, Das CK, Ghanta S (2021) Design of CHNO based new hetero-cyclic high energy density molecules: a theoretical survey. Struct Chem 32:1095–1104
Volokhov VM, Amosova ES, Volokhov AV, Zyubina TS, Lempert DB, Yanovskiy LS, Fateev ID (2020) Computer design of structure of molecules of high-energy tetrazines. Calculation of Thermochemical Properties. Supercomput Front Innov 7:68–79
Ameen R, Biju AR, Fasila PM (2021) Theoretical studies of azete based high energy density materials with trinitromethane functional group. Comput Theor Chem 1203:113346
Ameen R, Fasila PM, Anoop A, Biju AR (2021) Detonation properties and impact sensitivities of trinitromethane derivatives of three-membered heterocyclic ring compounds. J Mol Graph Model 105:107863
Thottempudi V, Gao H, Shreeve JNM (2011) Trinitromethyl-substituted 5-nitro-or 3-azo-1,2,4-triazoles:synthesis, characterization, and energetic properties. J Am Chem Soc 133:6464–6471
Zhang Y, Li Y, Hu J, Ge Z, Sun C, Pang S (2019) Energetic C-trinitromethyl-substituted pyrazoles: synthesis and characterization. Dalton Trans 48:1524–1529
Li Y, Xia H, Song S, Wang K, Zhang Q (2023) One-step synthesis of 6-amino-5-nitro-2-(trinitromethyl)-pyrimidin-4 (3H)-one as potential energetic material. Inorg Chem Commun 152:110673
Sun CH, Zhao XQ, Li YC, Pang SP (2010) Synthesis of two new cage molecules containing trinitromethyl group. Chin Chem Lett 21:572–575
Xu Y, Shen C, Lin Q, Wang P, Jiang C, Lu M (2016) 1-Nitro-2-trinitromethyl substituted imidazoles: a new family of high performance energetic materials. J Mater Chem A 4:17791–17800
Calais JL (1993) Density-functional theory of atoms and molecules. Int J Quantum Chem 47:101
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H et al (2009) Gaussian 09; Gaussian Inc, Wallingford. CT 32:5648–5652
Miehlich V, Savin A, Stoll V, Preuss V (1989) Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett 157:200–206
Ravi P, Tewari SP (2012) A DFT study on the structure-property relationship of amino-nitro-and nitrosotetrazoles, and their N-oxides: new high energy density molecules. Struct Chem 23:487–498
Ma Q, Jiang T, Zhang X, Fan G, Wang J, Huang J (2015) Theoretical investigations on 4, 4′, 5, 5′ -tetranitro-2, 2′ -1H, 1′ H-2, 2′ -biimidazole derivatives as potential nitrogen-rich high energy materials. J Phys Org Chem 28:31–39
Ravi P, Tewari SP, Ramaswamy R (2013) A DFT Study on the structures and energies of isomers of 4-amino-1, 3-dinitro-1, 2, 4-triazol-5-one-2-oxide: new high energy density compounds. Propellants Explos Pyrotech 38:425–432
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Fasila PM, Rahana A, Biju AR (2021) Theoretical investigation of energetic performance and impact sensitivities of nitro and trinitromethyl substituted ozonides of ethylene and cyclopentene. Comput Theor Chem 1205:113425
Politzer P, Murray JS, Edward Grice M, Desalvo M, Miller E (1997) Calculation of heats of sublimation and solid phase heats of formation. Mol Phys 91:923–928
Politzer P, Murray JS (2011) Some perspectives on estimating detonation properties of C, H, N, O compounds. Cent Eur J Energ Mater 8:209–220
Ravi P, Gore GM, Venkatesan V, Tewari SP, Sikder AK (2010) Theoretical studies on the structure and detonation properties of amino-, methyl-, and nitro-substituted 3,4,5-trinitro-1H-pyrazoles. J Hazard Mater 183:1–7
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C-H-N-O explosives. J Chem Phys 48:23–35
KamletMJ AHG (1979) The relationship of impact sensitivity with structure of organic high explosives. II. Polynitroaromatic explosives. Propellants Explos Pyrotech 4:30–34
Rice BM, Sahu S, Owens FJ (2002) Density functional calculations of bond dissociation energies for NO2 scission in some nitroaromatic molecules. J Mol Struct Theochem 583:69–72
Pospišil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16:895–901
Akhavan J (2011) The chemistry of explosives. The Royal Society of Chemistry, Cambridge, UK
Urbanski T (1964) Chemistry and technology of explosives. Pergamon, Oxford, UK
Murray JS, Lane P, Göbel M, Klapötke TM, Politzer P (2009) Intra-and intermolecular electrostatic interactions and their significance for the structure, acidity, and tautomerization behavior of trinitromethane. J Chem Phys 130:104304
Göbel M, Tchitchanov B, Murray J et al (2009) Chlorotrinitromethane and its exceptionally short carbon–chlorine bond. Nature Chem 1:229–235
Macaveiu L, GöbelM KTM et al (2010) The unique role of the nitro group in intramolecular interactions: chloronitromethanes. Struct Chem 21:139–146
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Schmalz TG, SeitzWA KDJ, Hite GE (1988) Elemental carbon cages. J Am Chem Soc 110:1113–1127
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Wiley Interdiscip Rev Comput Mol Sci 1:153–163
Zhao G, Lu M (2014) Comparative theoretical studies of high energetic cyclic nitramines. J Phys Org Chem 27:10–17
Hammerl A, Klapötke TM, NöthH WM, Holl G (2003) Synthesis, structure, molecular orbital and valence bond calculations for tetrazole azide, CHN7, Propellants. Explos Pyrotech An Int J Deal with Sci Technol Asp Energ Mater 28:165–173
Trache D, Klapötke TM, Maiz L, Abd-Elghany M, DeLuca LT (2017) Recentadvances in new oxidizers for solid rocket propulsion. Green Chem 19:4711–4736
Lysien K, Stolarczyk A, Jarosz T (2021) Solid propellant formulations: A review of recent progress and utilized components. Materials 14:6657
Mader CL (1961) Detonation performance calculations using the Kistiakowsky-Wilson equation of state, Los Alamos Scientific Laboratory of the University of California
Owens FJ (1996) Calculation of energy barriers for bond rupture in some energetic molecules. J Mol Struct Theochem 370:11–16
Ou YX, Liu JQ (2005) High energy density compounds. Natl Def Ind Press Beijing 20:25–30
Yongjin C, Shuhong B (2019) High energy density material (HEDM)-progress in research azine energetic compounds. Johns Matthey Technol Rev 63:51–72
Lin H, Zhu Q, Huang C, Yang DD, Lou N, Zhu SG, Li HZ (2019) Dinitromethyl, fluorodinitromethyl derivatives of RDX and HMX as high energy density materials: a computational study. Struct Chem 30:2401–2408
PolitzerP MJS (2015) Some molecular/crystalline factors that affect the sensitivities of energetic materials: molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J Mol Model 21:1–11
Rice BM, HareJJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106:1770–1783
Talawar MB, Sivabalan R, Mukundan T, Muthurajan H, Sikder AK, GandheBR RAS (2009) Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater 161:589–607
Acknowledgements
RA thanks Dr. Fasila P. M, Assistant Professor of the Department of PG Studies and Research in Chemistry, Sir Syed College, in Taliparamba for her wholehearted help for the completion of this work.
Author information
Authors and Affiliations
Contributions
ARB: designing of work, revised draft and approved. RA drafted the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ameen, R., Biju, A.R. Theoretical study of a series of 1,2-diazete based trinitromethyl derivatives as potential energetic compounds. J Mol Model 30, 178 (2024). https://doi.org/10.1007/s00894-024-05971-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05971-8