Abstract
Context
1,3-Propanediol (1,3-PDO) is a key chemical in various industries, including pharmaceuticals and material sciences, and is projected to see significant market growth. However, the current challenges in its downstream processing, particularly in terms of cost and efficiency, highlight the need for innovative solutions. Our study delves into using ionic liquids (ILs) as a potential alternative, aiming to address these critical separation challenges more sustainably and efficiently.
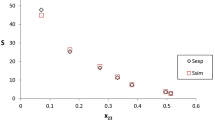
In this study, we utilized molecular dynamics (MD) simulations and the COSMO-SAC to examine 1,3-propanediol (1,3-PDO) extraction using four imidazolium-based ionic liquids with 1-butyl-3-methylimidazolium [Bmim] cation and with different anions bis(pentafluoroethanesulfonyl)imide [NPF2]−, bis(trifluoromethylsulfonyl)imide [NTF2]−, thiocyanate [SCN]−, and trifluoromethanesulfonate [TFO]−. Molecular dynamics simulations, incorporating analysis of radial distribution functions (RDF) and spatial distribution functions (SDF), revealed that [Bmim][SCN] and [Bmim][TFO] exhibit enhanced interactions with 1,3-PDO. Notably, [Bmim][SCN] formed the most hydrogen bonds, averaging 1.639 per molecule, due to its coordinating [SCN]− anion. This was in contrast to the fewer hydrogen bonds formed by non-coordinating anions in [Bmim][NPF2] and [Bmim][NTF2]. In ternary systems, [Bmim][SCN] and [Bmim][TFO] demonstrated superior selectivity for 1,3-PDO extraction compared to the other ionic liquids, with selectivity values around 29. These findings, supported by COSMO-SAC predictive modeling, highlight the potential of [Bmim][SCN] as a promising candidate for 1,3-PDO extraction, emphasizing the importance of anion selection in optimizing ionic liquid properties for this application.
Methods
In our study, we employed MD simulations, incorporating the OPLS-AA force field, and COSMO-SAC to investigate the extraction of 1,3-PDO using imidazolium-based ionic liquids: [Bmim][NTF2], [Bmim][NPF2], [Bmim][SCN], and [Bmim][TFO]. The MD simulations were conducted using LAMMPS software, focusing on elucidating the RDF, SDF, and hydrogen bonding. Analysis of the distribution coefficient (β) and selectivity (S) for the ternary mixture was also conducted. These aspects of the simulation were analyzed using TRAVIS and VMD software. Additionally, the COSMO-SAC model was employed to determine the activity coefficients of 1,3-PDO in the ionic liquids, with molecular optimization conducted using Gaussian16 and sigma profile calculations performed using COSMO-SAC.
Graphical Abstract











Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Saxena RK, Anand P, Saran S, Isar J (2009) Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnol Adv 27(6):895–913
da Silva Ruy AD, de Brito Alves RM, Reis Hewer TL, de Aguiar PD, Gomes Teixeira LS, Magalhães Pontes LA (2021) Catalysts for glycerol hydrogenolysis to 1,3-propanediol: a review of chemical routes and market. Catal Today 1(381):243–253
Kurian JV (2005) A new polymer platform for the future - Sorona® from corn derived 1,3-propanediol. J Polym Environ 13(2):159–167
Xiu ZL, Zeng AP (2008) Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl Microbiol Biotechnol 78:917–26
Gao S, Zhang D, Sun Y, Xiu Z (2007) Separation of 1,3-propanediol from glycerol-based fermentations of Klebsiella pneumoniae by alcohol precipitation and dilution crystallization. Front Chem Eng China 1(2):202–7. https://doi.org/10.1007/s11705-007-0037-1
(1) Desalination of fermented broth containing 1,3-propanediol by electrodialysis [Internet]. [cited 2024 Mar 22]. https://www.researchgate.net/publication/279701849_Desalination_of_fermented_broth_containing_13-propanediol_by_electrodialysis
Maddox IS (1989) The acetone-butanol-ethanol fermentation: recent progress in technology. Biotechnol Genet Eng Rev [Internet]. [cited 2024 Mar 23];7(1):189–220. https://pubmed.ncbi.nlm.nih.gov/2696472/
Hao J, Sun Y, Wang Q, Tong X, Zhang H, Zhang Q (2010) Effect and mechanism of penetration enhancement of organic base and alcohol on glycyrrhetinic acid in vitro. Int J Pharm 399(1–2):102–108
Boonoun P, Laosiripojana N, Muangnapoh C, Jongsomjit B, Panpranot J, Mekasuwandumrong O et al (2010) Application of sulfonated carbon-based catalyst for reactive extraction of 1,3-propanediol from model fermentation mixture. Ind Eng Chem Res 49(24):12352–12357
Hao J, Liu H, Liu D (2005) Novel route of reactive extraction to recover 1,3-propanediol from a dilute aqueous solution. Ind Eng Chem Res 44(12):4380–4385
Hilaly AK, Binder TP (2002) U.S. patent no. 6,479,716. Washington, DC: U.S. Patent and trademark office
Gao S, Zhang D, Sun Y, Xiu Z (2007) Separation of 1,3-propanediol from glycerol-based fermentations of Klebsiella pneumoniae by alcohol precipitation and dilution crystallization. Front Chem Eng China 1(2):202–207
Anand P, Saxena RK, Marwah RG (2011) A novel downstream process for 1,3-propanediol from glycerol-based fermentation. Appl Microbiol Biotechnol 90(4):1267–1276
Barski P, Kowalczyk J, Lindstaedt A, Puzewicz-Barska J, Witt D (2012) Evaluation of solid phase extraction for downstream separation of propane-1,3-diol and butan-1-ol from fermentation broth. Process Biochem 47(6):1005–1010
Cui c, Zhang Z, Chen B (2017) Environmentally-friendly strategy for separation of 1,3-propanediol using biocatalytic conversion. Bioresour Technol 245:477–82
Antony FM, Pal D, Wasewar K (2021) Separation of bio-products by liquid-liquid extraction. Physical Sciences Reviews 6(4):1–21. https://doi.org/10.1515/psr-2018-0065/html
Wischral D, Fu H, Pellegrini Pessoa FL, Pereira N, Yang ST (2018) Effective and simple recovery of 1,3-propanediol from a fermented medium by liquid–liquid extraction system with ethanol and K3PO4. Chin J Chem Eng 26(1):104–108
Boonsongsawat T, Shotipruk A, Tantayakom V, Prasitchoke P, Chandavasu C, Boonnoun P et al (2010) Solvent extraction of biologically derived 1,3-propanediol with ethyl acetate and ethanol cosolvent. Sep Sci Technol 45(4):541–547
Reichardt C, Welton T (2010) Solvents and solvent effects in organic chemistry: fourth edition. Solvents and solvent effects in organic chemistry: fourth edition [Internet]. 2010 Nov 26 [cited 2024 Mar 22]. https://onlinelibrary.wiley.com/doi/book/10.1002/9783527632220
Baniel AM, Jansen RP, Vitner A, Baiada A (2006) U.S. patent no. 7,056,439. Washington, DC: U.S. Patent and trademark office
Cho M-H, Joen SI, Pyo S-H, Mun S, Kim J-H (2006) A novel separation and purification process for 1,3-propanediol. Process Biochemistry 41(3):739–744. https://doi.org/10.1016/j.procbio.2005.11.013
Anvari M, Pahlavanzadeh H, Vasheghani-Farahani E, Khayati G (2009) In situ recovery of 2,3-butanediol from fermentation by liquid-liquid extraction. J Ind Microbiol Biotechnol 36:313–317. https://doi.org/10.1007/s10295-008-0501-z
Zhang J, Hu B (2013) Liquid-Liquid Extraction (LLE). Sep Purif Technol in Biorefineries 1:61–78
Zhou X, Zhou X, Wang C, Zhou H (2023) Environmental and human health impacts of volatile organic compounds: a perspective review. Chemosphere 1(313):137489
Singh SK, Savoy AW (2020) Ionic liquids synthesis and applications: an overview. J Mol Liq 1(297):112038
Kaur G, Kumar H, Singla M (2022) Diverse applications of ionic liquids: a comprehensive review. J Mol Liq 1(351):118556
Rajyaguru YV, Patil JH, Kusanur R (2022) Ionic liquids, an asset in extraction techniques–a comprehensive review. Rev Adv Chem 12(2):107–22. https://doi.org/10.1134/S2634827622020040
Lei Z, Chen B, Koo YM, Macfarlane DR (2017) Introduction: ionic liquids. Chem Rev 117(10):6633–5. https://doi.org/10.1021/acs.chemrev.7b00246
Marsh KN, Boxall JA, Lichtenthaler R (2004) Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilib 219(1):93–98
Wasserscheid P, Welton T (2008) Ionic liquids in synthesis: second edition. Ionic liquids in synthesis second edition 1:1–721. https://doi.org/10.1002/9783527621194
Chapeaux A, Simoni LD, Ronan TS, Stadtherr MA, Brennecke JF (2008) Extraction of alcohols from water with 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. Green Chem [Internet]. (12):1301–6. https://pubs.rsc.org/en/content/articlehtml/2008/gc/b807675h
Ha SH, Mai NL, Koo YM (2010) Butanol recovery from aqueous solution into ionic liquids by liquid-liquid extraction. Process Biochem 45(12):1899–1903
Nann A, Held C, Sadowski G (2013) Liquid-liquid equilibria of 1-butanol/water/IL systems. Ind Eng Chem Res 52(51):18472–18481
Ha SH, Mai NL, Koo YM (2010) Butanol recovery from aqueous solution into ionic liquids by liquid–liquid extraction. Process Biochem 45(12):1899–1903
Yang J, Luo K, Lu X, He W, Zhao S, Fang Z et al (2023) Selective extraction of polyhydroxy compounds using hydrophobic ionic liquids. Sep Purif Technol 1(318):123973
Cháfer A, de la Torre J, Loras S, Montón JB (2018) Study of liquid–liquid extraction of ethanol + water azeotropic mixtures using two imidazolium-based ionic liquids. J Chem Thermodyn 1(118):92–99
Neves CMSS, Granjo JFO, Freire MG, Robertson A, Oliveira NMC, Coutinho JAP (2011) Separation of ethanol–water mixtures by liquid–liquid extraction using phosphonium-based ionic liquids. Green Chem 13(6):1517–1526
Shen Y, Xu Y, Meng D, Chen Z, Li H, Zhang Y et al (2021) Molecular mechanism and extraction performance evaluation of ionic liquids for extraction process of n-heptane/n-propanol. Sep Purif Technol 1(276):119342
Domańska U, Wlazło M, Paduszyński K (2018) Extraction of butan-1-ol from aqueous solution using ionic liquids: an effect of cation revealed by experiments and thermodynamic models. Sep Purif Technol 8(196):71–81
Dezhang S, Huisheng F, Feng X, Wenxiu L, Zhigang Z (2019) Feasibility of ionic liquid as extractant for bio-butanol extraction: experiment and simulation. Sep Purif Technol 15(215):287–298
Zhu Z, Xu Y, Li H, Shen Y, Meng D, Cui P et al (2020) Separation of isopropyl alcohol and isopropyl ether with ionic liquids as extractant based on quantum chemical calculation and liquid-liquid equilibrium experiment. Sep Purif Technol 247:116937. https://doi.org/10.1016/j.seppur.2020.116937
Mosallanejad MR, Khosravi-Nikou MR, Shariati A (2018) Separation of ethanol from n-decane-ethanol mixtures using imidazolium based ionic liquids. J Chem Thermodyn 131:471–477. https://doi.org/10.1016/j.jct.2018.11.027
Garcia-Chavez LY, Shazad M, Schuur B, De Haan AB (2012) (Liquid + liquid) equilibrium data for the separation of 2,3-butanediol from aqueous streams using tetraoctyl ammonium 2-methyl-1-naphthoate. J Chem Thermodyn 1(55):85–91
Liu XH, Rebroš M, Dolejš I, Marr AC (2017) Designing ionic liquids for the extraction of alcohols from fermentation broth: phosphonium alkanesulfonates, solvents for diol extraction. ACS Sustain Chem Eng 5(9):8260–8268
Nian B, Cao C, Liu Y (2019) Lipase and metal chloride hydrate-natural deep eutectic solvents synergistically catalyze amidation reaction via multiple noncovalent bond interactions. ACS Sustain Chem Eng 7(21):18174–84. https://doi.org/10.1021/acssuschemeng.9b05691
Zhu Z, Bai W, Xu Y, Gong H, Wang Y, Xu D et al (2019) Liquid-liquid extraction of methanol from its mixtures with hexane using three imidazolium-based ionic liquids. J Chem Thermodyn 1(138):189–195
Naik PK, Mohan M, Banerjee T, Paul S, Goud VV (2018) Molecular dynamic simulations for the extraction of quinoline from heptane in the presence of a low-cost phosphonium-based deep eutectic solvent. J Phys Chem B 122(14):4006–4015
Zhu Z, Li H, Xu Y, Zhang W, Shen Y, Gao J et al (2020) Quantum chemical calculation, molecular dynamics simulation and process design for separation of heptane - butanol using ionic liquids extraction. J Mol Liq 10(316):113851
Wang X, Gu X, Murad S (2018) Molecular dynamics simulations of liquid-liquid phase equilibrium of ternary methanol/water/hydrocarbon mixtures. Fluid Phase Equilib 25(470):109–119
Li J, Wang J, Wu M, Cheng H, Chen L, Qi Z (2020) Deep deterpenation of citrus essential oils intensified by in situ formation of a deep eutectic solvent in associative extraction. Ind Eng Chem Res 59:9223
Lin ST, Sandler SI (2001) A priori phase equilibrium prediction from a segment contribution solvation model. Ind Eng Chem Res 41(5):899–913. https://doi.org/10.1021/ie001047w
Xiong R, Sandler SI, Burnett RI (2014) An improvement to COSMO-SAC for predicting thermodynamic properties. Ind Eng Chem Res 53(19):8265–8278
Chen WL, Hsieh CM, Yang L, Hsu CC, Lin ST (2016) A critical evaluation on the performance of COSMO-SAC models for vapor-liquid and liquid-liquid equilibrium predictions based on different quantum chemical calculations. Ind Eng Chem Res 55(34):9312–22. https://doi.org/10.1021/acs.iecr.6b02345
Mullins E, Oldland R, Liu YA, Wang S, Sandler SI, Chen CC et al (2006) Sigma-profile database for using COSMO-based thermodynamic methods. Ind Eng Chem Res 45(12):4389–4415
Meng X, Li R, Bing X, Gao J, Xu D, Zhang L et al (2020) Liquid-liquid equilibrium measurements and interaction exploration for separation of isobutyl alcohol + isobutyl acetate by imidazolium-based ionic liquids with different anions. J Chem Thermodyn 1(141):105932
Shah MR, Yadav GD (2011) Prediction of liquid-liquid equilibria for biofuel applications by quantum chemical calculations using the Cosmo-SAC method. Ind Eng Chem Res 50(23):13066–75. https://doi.org/10.1021/ie201454m
Zhang L, Zhang M, Gao J, Xu D, Zhou S, Wang Y (2018) Efficient extraction of neutral heterocyclic nitrogen compounds from coal tar via ionic liquids and its mechanism analysis. Energy Fuels 32(9):9358–70. https://doi.org/10.1021/acs.energyfuels.8b02297
Bharti A, Verma R, Prerna, Sarvesh Namdeo, Malviya A, Banerjee T, et al (2018) Liquid-liquid equilibria and COSMO-SAC modeling of organic solvent/ionic liquid - hydroxyacetone - water mixtures. Fluid Phase Equilib 462:73–84
Tokuda H, Tsuzuki S, Susan MABH, Hayamizu K, Watanabe M (2006) How ionic are room-temperature ionic liquids? An indicator of the physicochemical properties. J Phys Chem B 110(39):19593–19600
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. Journal of the American Chemical Society 118(45):11225–11236. https://pubs.acs.org/sharingguidelines
Doherty B, Zhong X, Gathiaka S, Li B, Acevedo O (2017) Revisiting OPLS force field parameters for ionic liquid simulations. J Chem Theory Comput 13(12):6131–6135
Udier-Blagović M, Morales De Tirado P, Pearlman SA, Jorgensen WL (2004) Accuracy of free energies of hydration using CM1 and CM3 atomic charges. J Comput Chem 25(11):1322–32. https://doi.org/10.1002/jcc.20059
Dodda LS, Cabeza De Vaca I, Tirado-Rives J, Jorgensen WL (2017) LigParGen web server: an automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res [Internet]. [cited 2023 Jul 12];45:331–6. https://academic.oup.com/nar/article/45/W1/W331/3747780
Dodda LS, Vilseck JZ, Tirado-Rives J, Jorgensen WL (2017) 1.14∗CM1A-LBCC: localized bond-charge corrected CM1A charges for condensed-phase simulations. J Phys Chem B 121(15):3864–70. https://doi.org/10.1021/acs.jpcb.7b00272
Vassetti D, Pagliai M, Procacci P (2019) Assessment of GAFF2 and OPLS-AA general force fields in combination with the water models TIP3P, SPCE, and OPC3 for the solvation free energy of druglike organic molecules. J Chem Theory Comput 15(3):1983–95. https://doi.org/10.1021/acs.jctc.8b01039
Martinez L, Andrade R, Birgin EG, Martínez JM (2009) PACKMOL: a package for building initial configurations for molecular dynamics simulations. J Comput Chem 30(13):2157–2164
Li K, Lin X, Greenberg J (2016) Software citation, reuse and metadata considerations: an exploratory study examining LAMMPS. Proc Assoc Inf Sci Technol 53(1):1–10
Yue K, Doherty B, Acevedo O (2022) Comparison between ab initio molecular dynamics and OPLS-based force fields for ionic liquid solvent organization. J Phys Chem B 126(21):3908–3919
Luty BA, Davis ME, Tironi IG, Van Gunsteren WF (1994) A Comparison of particle-particle, particle-mesh and Ewald methods for calculating electrostatic interactions in periodic molecular systems. Mol Simul 14(1):11–20. https://doi.org/10.1080/08927029408022004
Nosé S, Klein ML (1983) A study of solid and liquid carbon tetrafluoride using the constant pressure molecular dynamics technique. J Chem Phys 78(11):6928–39
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31(3):1695. https://doi.org/10.1103/PhysRevA.31.1695
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52(12):7182–90
Brehm M, Thomas M, Gehrke S, Kirchner B (2020) TRAVIS—a free analyzer for trajectories from molecular simulation. J Chem Phys 152(16):164105
Humphrey W, Dalke A, Schulten K (1996) VMD – visual molecular dynamics. J Mol Graph 14:33–38
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–35
Vega C, Abascal JLF (2011) Simulating water with rigid non-polarizable models: a general perspective. Physical Chemistry Chemical Physics 13(44):19663–88
Mark P, Nilsson L (2001) Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J Phys Chem A 105(43):9954–60. https://doi.org/10.1021/jp003020w
Working with water in LAMMPS - Christopher O’Brien, Ph.D. [Internet]. [cited 2023 Sep 27]. https://sites.google.com/a/ncsu.edu/cjobrien/tutorials-and-guides/working-with-water-in-lammps
Klamt A (2002) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99(7):2224–35. https://doi.org/10.1021/j100007a062
Klamt A, Jonas V, Bürger T, Lohrenz JCW (1998) Refinement and parametrization of COSMO-RS. J Phys Chem A 102(26):5074–85. https://doi.org/10.1021/jp980017s
Klamt A, Eckert F (2000) COSMO-RS: a novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib 172(1):43–72
Klamt A, Lin ST, Sandler SI (2002) Comments on ‘A priori phase equilibrium prediction from a segment contribution solvation model’ (Multiple letters). Ind Eng Chem Res 41(9):2330–4. https://doi.org/10.1021/ie011031l
Hanwell MD, Curtis DE, Lonie DC, Vandermeerschd T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform [Internet]. [cited 2023 Oct 30] 4(1). https://pubmed.ncbi.nlm.nih.gov/22889332/
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb M, Cheeseman JR, Scalmani G, Barone V, Petersson G, Nakatsuji H, Li X, Caricato M, Marenich V, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov F, Sonnenberg JL, Williams Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery J Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell P, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) G16_C01
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–52
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (2002) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem® 98(45):11623–7. https://doi.org/10.1021/j100096a001
Anantharaj R, Banerjee T (2010) COSMO-RS-based screening of ionic liquids as green solvents in denitrification studies. Ind Eng Chem Res 49(18):8705–25. https://doi.org/10.1021/ie901341k
Bell IH, Mickoleit E, Hsieh CM, Lin ST, Vrabec J, Breitkopf C et al (2020) A benchmark open-source implementation of COSMO-SAC. J Chem Theory Comput 16(4):2635–46. https://doi.org/10.1021/acs.jctc.9b01016
Doherty B, Zhong X, Gathiaka S, Li B, Acevedo O (2017) Revisiting OPLS force field parameters for ionic liquid simulations. J Chem Theory Comput 13(12):6131–6135
Tokuda H, Ishii K, Susan MABH, Tsuzuki S, Hayamizu K, Watanabe M (2006) Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures. J Phys Chem B 110(6):2833–9
Yee P, Shah JK, Maginn EJ (2013) State of hydrophobic and hydrophilic ionic liquids in aqueous solutions: are the ions fully dissociated? J Phys Chem B 117(41):12556–12566
Gonfa G, Bustam MA, Muhammad N, Khan AS (2015) Evaluation of thermophysical properties of functionalized imidazolium thiocyanate based ionic liquids. Ind Eng Chem Res 54(49):12428–12437
Zhang R, Yue X, Li B, Yang J, Wu Z, Zhang J (2020) Dynamic viscosity, density and surface tension of 1, 3-propanediol (1) + 1,2-propanediamine (2) binary system at T = (293.15 to 318.15) K and atmosphere pressure. J Mol Liq 299:112213. https://doi.org/10.1016/j.molliq.2019.112213
Zhao Y, Gao S, Wang J, Tang J (2008) Aggregation of ionic liquids [Cnmim]Br (n = 4, 6, 8, 10, 12) in D2O: a NMR study. J Phys Chem B 112(7):2031–9. https://doi.org/10.1021/jp076467e
Molecular Simulation/Radial Distribution Functions - Wikibooks, open books for an open world [Internet]. [cited 2023 Sep 16]. https://en.wikibooks.org/wiki/Molecular_Simulation/Radial_Distribution_Functions
Paschek D, Golub B, Ludwig R (2015) Hydrogen bonding in a mixture of protic ionic liquids: a molecular dynamics simulation study. Phys Chem Chem Phys [Internet]. 17(13):8431–40. https://pubs.rsc.org/en/content/articlehtml/2015/cp/c4cp05432f
Zhu Z, Li H, Xu Y, Zhang W, Shen Y, Gao J et al (2020) Quantum chemical calculation, molecular dynamics simulation and process design for separation of heptane - butanol using ionic liquids extraction. J Mol Liq 10(316):113851
Weber H, Hollóczki O, Pensado AS, Kirchner B (2013) Side chain fluorination and anion effect on the structure of 1-butyl-3-methylimidazolium ionic liquids. J Chem Phys [Internet]. [cited 2024 Mar 23] 139(8). https://pubmed.ncbi.nlm.nih.gov/24007013/
Kempter V, Kirchner B (2010) The role of hydrogen atoms in interactions involving imidazolium-based ionic liquids. J Mol Struct 972(1–3):22–34
Peppel T, Roth C, Fumino K, Paschek D, Köckerling M, Ludwig R (2011) The influence of hydrogen-bond defects on the properties of ionic liquids. Angew Chem 50(29):6661–6665
Kumar N, Naik PK, Banerjee T (2020) Molecular modeling insights in the extraction of benzene from hydrocarbon stream using deep eutectic solvent. J Mol Liq 1(317):113909
Paul S, Paul S (2013) The influence of trehalose on hydrophobic interactions of small nonpolar solute: a molecular dynamics simulation study. J Chem Phys [Internet]. [cited 2023 Sep 27] 139(4). https://pubmed.ncbi.nlm.nih.gov/23901994/
Pethes I, Bakó I, Pusztai L (2020) Chloride ions as integral parts of hydrogen bonded networks in aqueous salt solutions: the appearance of solvent separated anion pairs. Phys Chem Chem Phys 22(19):11038–11044
Domańska U, Królikowska M (2010) Effect of temperature and composition on the surface tension and thermodynamic properties of binary mixtures of 1-butyl-3-methylimidazolium thiocyanate with alcohols. J Colloid Interface Sci 348(2):661–667
Pereiro AB, Rodríguez A (2007) Study on the phase behaviour and thermodynamic properties of ionic liquids containing imidazolium cation with ethanol at several temperatures. J Chem Thermodyn 39(6):978–989
Acknowledgements
The authors express gratitude to Birla Institute of Technology, Mesra, for their support and resources in conducting this research. Author(s) acknowledge the Department of Computer Science and Engineering, BIT-MESRA, for providing a High Performance Computing facility to carry out the research.
Author information
Authors and Affiliations
Contributions
"Raj Akshat is the Ph.D. scholar, is the primary author who has undertaken the majority of the writing and analysis for the manuscript. Anand Bharti and Padmini Padmanabhan, both corresponding authors, have significantly contributed to developing the research idea and have provided extensive mentorship and teaching to a. throughout the research process. Anand Bharti and Padmini Padmanabhan. also played key roles in refining the study's methodology and in critically reviewing and editing the manuscript. All authors have collaboratively reviewed the final manuscript and approved the final version for publication. Raj Akshat is identified as the Ph.D. scholar mainly responsible for writing and analysis. Anand Bharti and Padmini Padmanabhan are corresponding authors who have not only mentored the main author but also contributed to the conception and development of the research idea. The collaborative nature of the work, including the review and approval of the final manuscript by all authors, is emphasized.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akshat, R., Bharti, A. & Padmanabhan, P. Atomistic molecular dynamics simulation and COSMO-SAC approach for enhanced 1,3-propanediol extraction with imidazolium-based ionic liquids. J Mol Model 30, 164 (2024). https://doi.org/10.1007/s00894-024-05964-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05964-7