Abstract
Context
Xanthates are organic compounds of great interest in coordination chemistry due to their different basic sites, which allow them to form complexes with different coordination modes and geometries. These compounds are relevant in the environment and act as heavy metal collectors in aqueous environments. In this theoretical–experimental work, electronic spectroscopy studies of n-propyl xanthate complexes with group 12 metals were performed. This study verified structural differences in these systems, depending on the environment in which they are inserted. In addition, structural differences were observed when the solid was changed to an n-hexane solution. Thus, it was observed that the complexes assume a mononuclear structure in solution, while they present a polymeric form in the solid phase. The electronic spectra obtained through TD-DFT calculations were compared to those of the previously synthesized complexes. In the final theoretical analysis, the main orbitals involved in these transitions were assigned using population analysis calculations. The synthesis of the complexes was confirmed through infrared (MID and FAR), UV‒Vis, Raman, and NMR-1H spectroscopic analyses.
Methods
The structures of the mononuclear and polymeric complexes were optimized in vacuum and n-hexane. Under vacuum, DFT levels M06L/6–311 + + G** + LANL2TZ and M06L/def2-TZVP were used for the mononuclear complexes, and M06L/LANL2DZ + LANL2 were used for the polymer complexes. For the calculations of the mononuclear complexes in n-hexane, the same level of theory was used for the solid state. TD-DFT calculations for 300 excited states were performed with the same levels of theory and used the optimized structures of the complexes. Furthermore, population analysis was carried out on all the systems studied. Gaussian 09 software was used for the structure optimization, TD-DFT, and population analysis calculations. GaussSum software was used to evaluate the molecular orbitals and electronic spectra.
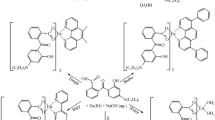
Graphical Abstract






Similar content being viewed by others
Data availability
This published article and its supplementary information file include all the data generated or analyzed during this study.
References
Werner A (1893) Beitrag zur Konstitution anorganischer Verbindungen. Z Anorg Chem 3:267–330. https://doi.org/10.1002/zaac.18930030136
Ruixuan Q, Kunlong L, Qingyan W, Zheng N (2020) Surface coordination chemistry of atomically dispersed metal catalysts. Chem Rev 120:11810–11899. https://doi.org/10.1021/acs.chemrev.0c00094
Smithells A (1906) Neuere anschauungen auf dem gebiete der anorganischen chemie. Nature 73:433–434. https://doi.org/10.1038/073433a0
Busch DH (1993) The complete coordination chemistry—one practioner’s perspective. Chem Rev 93:847–860. https://doi.org/10.1021/cr00019a001
Chen Z, Deutsch TG, Dinh HN, Domen K, Emery K, Forman AJ, Gaillard N, Garland R, Heske C, Jaramillo TF, Kleiman-Shwarsctein A, Miller E, Takanabe K, Turner J (2013) Photoelectrochemical water splitting. Energy book series. UV-Vis spectroscopy. Springer Briefs, New York, pp 49–61
Rocha FS, Gomes AJ, Lunardi CN, Kaliaguine S, Patience GS (2018) Experimental methods in chemical engineering: ultraviolet visible spectroscopy—UV-Vis. Can J Chem Eng 96:2512–2517. https://doi.org/10.1002/cjce.23344
Liu K, Shi W, Cheng P (2011) The coordination chemistry of Zn(II), Cd(II) and Hg(II) complexes with 1,2,4-triazole derivatives. Dalton Trans 40:8475–8490. https://doi.org/10.1039/c0dt01578d
Heering C, Francis B, Nateghi B, Makhloufi G, Ludeke S, Janiak C (2016) Syntheses, structures and properties of group 12 element (Zn, Cd, Hg) coordination polymers with a mixed-functional phosphonatebiphenyl-carboxylate linker. Cryst Eng Comm 18:5209–5223. https://doi.org/10.1039/C6CE00587J
Onwudiwe DC, Krüger TPJ, Strydom CA (2014) Laser assisted solid-state reaction for the synthesis of ZnS and CdS nanoparticles from metal xanthate. Mater Lett 116:154–159. https://doi.org/10.1016/j.matlet.2013.10.118
Friebolin W, Schilling G, Zöller M, Amtmann E (2005) Antitumoral activity of nonplatinum xanthate complexes. J Med Chem 48:7925–7931. https://doi.org/10.1021/jm040899l
Banti CN, Kourkoumelis N, Tsiafoulis CG, Skoulika S, Hadjikakou SK (2017) Silver(I) complexes of methyl xanthate against human adenocarcinoma breast cancer cells. Polyhedron 121:115–122. https://doi.org/10.1016/j.poly.2016.09.056
Qadir AM (2016) Synthesis, characterization and antibacterial activities of two nickel(II) complexes with xanthate derivatives and N, N, N′, N′-tetramethylethylenediamine as ligands. Transit Met Chem 42:35–39. https://doi.org/10.1007/s11243-016-0103-y
Chang Q, Hao X, Duan L (2008) Synthesis of crosslinked starch-graft-polyacrylamide-co-sodium xanthate and its performances in wastewater treatment. J Hazard Mater 159:548–553. https://doi.org/10.1080/17458080.2017.1321793
Miranda DB, Quintal SMO, Ferreira GB (2021) Applications of xanthate metal compounds in the last ten years: a review. Rev Virtual Quim. https://doi.org/10.21577/1984-6835.20220054
Amrollahi L, Massinaei M, Moghaddam ZA (2019) Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nanoparticles. Miner Eng 134:142–155. https://doi.org/10.1016/j.mineng.2019.01.031
Zeise WC (1834) Vorläufiger bericht von erneuten unter-suchungen über die xanthogen. Ann Phys Chem XXXII 108:305–307. https://doi.org/10.1002/andp.18341082002
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. In: Luch A (ed). Mol Clin Environ Toxicol 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Wang S, Zhang C, Chang Q (2017) Synthesis of magnetic crosslinked starch-graft-poly(acrylamide)-co-sodium xanthate and its application in removing heavy metal ions. J Exp Nanosci 12:270–284. https://doi.org/10.1080/17458080.2017.1321793
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23:783–792. https://doi.org/10.1007/s10534-010-9328-y
Mant TGK, Lewis JL, Mattoo TK, Rigden SPA, Volans GN, House IM, Wakefield AJ, Cole RS (1987) Mercury poisoning after disc-battery ingestion. Hum Toxicol 6:179–181. https://doi.org/10.1177/096032718700600212
Ashurst KG, Finkelstein NP, Rice NM (1970) Studies of cyanide–xanthate mixed ligand complexes of mercury(II) in aqueous solution. J Chem Soc A: Inorg Phys Theor 0:2302–2307. https://doi.org/10.1039/J19700002302
Essam S, Salah D, Hassan M, Ibrahim I (2024) Modified coprecipitated copper ferrite nanoparticles as a dual-agent for Pb2+ and Cd2+ ions removal and antibacterial treatment in wastewater. Egyp J Pure Appl Sci 62:43–64. https://doi.org/10.21608/ejaps.2024.249912.1078
Liang S, Guo X, Tian Q (2011) Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulfured orange peel. Desalination 275:212–216. https://doi.org/10.1016/j.desal.2011.03.001
Corminboeuf C, Tran F, Weber J (2006) The role of density functional theory in chemistry: some historical landmarks and applications to zeolites. J Mol Struct: Theochem 762:1–7. https://doi.org/10.1016/j.theochem.2005.07.036
Lewards EG (2011) Computational chemistry: Introduction to the theory and applications of molecular and quantum mechanics. Springer, Canada
Abd El-Lateef HM, Khalaf MM, Kandeel M, Amer AA, Abdelhamid AA, Abdou A (2023) Designing, characterization, biological, DFT, and molecular docking analysis for new FeAZD, NiAZD, and CuAZD complexes incorporating 1-(2-hydroxyphenylazo)−2-naphthol (H2AZD). Comp Biol Chem 105:107908. https://doi.org/10.1016/j.compbiolchem.2023.107908
Abd El Aleem M, Elhady O, Abdou A, Alhashmialameer D, Eskander TNA, Abu-Dief AM (2023) Development of new 2-(benzothiazol-2-ylimino)-2,3-dihydro-1H-imidazol-4-ol complexes as robust catalysts for the synthesis of thiazole 6-carbonitrile derivatives supported by DFT studies. J Mol Struct 1292:136188. https://doi.org/10.1016/j.molstruc.2023.136188
Murugan T, Venkatesh R, Geetha K, Abdou A (2023) Synthesis, spectral investigation, DFT, antibacterial, antifungal and molecular docking studies of Ni(II), Zn(II), Cd(II) complexes of tetradentate Schiff-base ligand. Asian J Chem 35:1509–1517. https://doi.org/10.14233/ajchem.2023.27808
Abd El-Lateef HM, Khalaf MM, Amer AA, Kandeel M, Abdelhamid AA, Abdou A (2023) Synthesis, characterization, antimicrobial, density functional theory, and molecular docking studies of novel Mn(II), Fe(III), and Cr(III) complexes incorporating 4-(2-hydroxyphenyl azo)-1-naphthol (Az). ACS Omega 8:25877–25891. https://doi.org/10.1021/acsomega.3c01413
Najar AM, Eswayah A, Moftah MB, Ruwida OMK, Bobtaina E, Najwa M, Elhisadi TA, Tahani A, Tawati SM, Khalifa AMM, Abdou A, DowAltome AE (2023) Rigidity and flexibility of pyrazole, s-triazole, and v-triazole derivative of chloroquine as potential therapeutic against COVID-19. J Med Chem Sci 6:2056–2084. https://doi.org/10.26655/JMCHEMSCI.2023.9.14
Shaaban S, Al-Faiyz YS, Alsulaim GM, Alaasar M, Amri N, Ba-Ghazal H, Al-Karmalawy AA, Abdou A (2023) Synthesis of new organoselenium-based succinanilic and maleanilic derivatives and in silico studies as possible SARS-CoV-2 main protease inhibitors. Inorganics 11:321. https://doi.org/10.3390/inorganics11080321
Wang Y, Jina X, Yub HS, Truhlarb DG, He X (2017) Revised M06-L functional for improved accuracy on chemical reaction barrier heights, noncovalent interactions, and solid-state physics. Proc Natl Acad Sci 114:8487–8492. https://doi.org/10.1073/pnas.1705670114
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2009) Gaussian 09, version G09 D; Gaussian, Inc., Wallingford CT.
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681. https://doi.org/10.1002/jcc.10189
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Miranda DB, Quintal S, Ferreira GB (2023) Theoretical studies of Zn2+ complexes with alkyl xanthate ligands: a thermochemical, electronic energy decomposition, and natural bond orbital analysis. J Mol Model 29:1–19. https://doi.org/10.1007/s00894-023-05604-6
Miranda DB, Quintal S, Ferreira GB (2024) Theoretical studies in heavy metal complexation: understanding the structural, thermochemical, and electronic aspects. J Braz Chem Soc 35:1–21. https://doi.org/10.21577/0103-5053.20230141
Melengate GS, Quattrociocchi D, Siqueira Júnior JM, Stoyanov SR, Costa LM, Ferreira GB (2019) DFT study of the interaction between the Ni2+ and Zn2+ metal cations and the 1,2-dithiolene ligands: electronic, geometric and energetic analysis. J Braz Chem Soc 30:1161–1177. https://doi.org/10.21577/0103-5053.20190011
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/B508541A
Coates RA, McNary CP, Boles GC, Berden G, Oomens J, Armentrout PB (2015) Structural characterization of gas-phase cysteine and cysteine methyl ester complexes with zinc and cadmium dications by infrared multiple photon dissociation spectroscopy. Phys Chem Chem Phys 17:25799–25808. https://doi.org/10.1039/C5CP01500F
Al-Fahdawi A, Alsalihi E (2018) Synthesis and characterization of iron(II), cobalt(II), nickel(II), copper(II) and zinc(II) complexes using diphenylmethyl xanthate ligand. Aro: Sci J Koya Univ 6:33–37. https://doi.org/10.14500/aro.10243
Davara D, Zorlu Y (2017) Group 12 metal coordination polymers built on a flexibe hexakis(3-pyridyloxy)cyclotriphosphazene ligand: effect of the central metal ions on the construction of coordination polymers. Polyhedron 127:1–8. https://doi.org/10.1016/j.poly.2017.01.040
Giribabu K, Suresh R, Manigandan R, Vijayaraj A, Prabu R, Narayanan V (2012) Cadmium sulphide nanorods: synthesis, characterization and their photocatalytic activity. Bull Korean Chem Soc 33:2910–2916. https://doi.org/10.5012/bkcs.2012.33.9.2910
Matsunaga Y, Fujisawa K, Ibi N, Amir N, Miyashita Y, Okamoto K (2005) Zn(II) complexes with aliphatic thiolates: (Et4N)[Zn(SAd)3] and (Et4N)2[{Zn(ScHex)2}2(μ-ScHex)2]. Bull Chem Soc Jpn 78:1285–1287. https://doi.org/10.1246/bcsj.78.1285
Bakly AAK, Collison D, Ahumada-Lazo R, Binks DJ, Smith M, Raftery J, Whitehead GFS, O’Brien P, Lewis DJ (2021) Synthesis, X-ray single-crystal structural characterization, and thermal analysis of Bis(O-alkylxanthato)Cd(II) and Bis(O-alkylxanthato) Zn(II) complexes used as precursors for Cadmium and Zinc sulfide thin films. Inorg Chem 60:7573–7583. https://doi.org/10.1021/acs.inorgchem.1c01110
Bahij EF, Ara I, Lachkar M, Larbi NB (2003) Synthesis and characterization of ethylxanthato complexes of zinc(II) with P-donor ligands. Transit Metal Chem 28:908–912. https://doi.org/10.1023/A:1026326404549
Drew MGB, Hasan M, Hobson RJ, Rice AD (1986) Reactions of [Zn{S2P(OR)2}2] with Nitrogen bases and the single-crystal X-ray structures of [Zn{S2P(OPri)2}2]. H2NCH2CH2NH2 and [Zn{S2P(OPri)2}2]NC5H5. Dalton Trans 5:1161–1166. https://doi.org/10.1039/DT9860001161
Corwin DT, Koch SA (1988) Crystal structures of Zn(SR)2 complexes: structural models for the proposed [Zn(cys-S)2(his)2] center in transcription factor IIIA and related nucleic acid binding proteins. Inorg Chem 27:493–496. https://doi.org/10.1021/ic00276a011
Ara I, Bahij FE (2004) Synthesis and crystal structure of a zinc tris(xanthate) anionic derivative with a diaminoacridine cation. Inorg Chem Comm 7:1091–1094. https://doi.org/10.1016/j.inoche.2004.07.013
Mautner FA, Morsy AM, Youssef A, Goher MAS (1997) Polymeric complexes of cadmium(II) bridged simultaneously by tetradentate picolinato and μ(1,1)-azido or μ(N, S)-thiocyanato anions. Synthesis and structural characterization of [Cd(picolinato)(N3)]n and [Cd(picolinato)(NCS)]n. Polyhedron 16:235–242. https://doi.org/10.1016/0277-5387(96)00274-4
Rietveld HM, Maslen EG (1965) The crystal structure of cadmium n-butyl xanthate. Acta Crystallogr 18:429–436. https://doi.org/10.1107/S0365110X65000956
Tiekink ERT, Winter G (1992) Inorganic xanthates: a structural perspective. J Rev Inorg Chem 12:183–302. https://doi.org/10.1515/REVIC.1992.12.3-4.183
Hounslow AM, Lincoln SF, Tiekink ERT (1989) Correlations between nuclear magnetic resonance spectra and crystal structure. A 13C nuclear magnetic resonance study in the solid-state of bis(xanthato) complexes of mercury(II); The crystal and molecular structure of bis(n-propyldithiocarbonato)mercury(II). J Chem Soc Dalton Trans 21:133–137. https://doi.org/10.1039/DT9890000233
Cox MJ, Tiekink ERT (1999) Structural diversity in mercury(II)bis(O-alkyldithiocarbonate) compounds. Z Kristallogr 214:486–491. https://doi.org/10.1524/zkri.1999.214.8.486
Chieh C, Moynihan KJ (1980) Xanthate and dithiocarbamate complexes of group IIB elements, and an interesting relationship between two mercury(II) ethylxanthate phases. Acta Crystallogr 36:1367–1371. https://doi.org/10.1107/S0567740880006097
Batsanov SS (2001) van der Waals radii of elements. Inorg Mater 37:871–885. https://doi.org/10.1023/A:1011625728803
Mansouri TH, Zareian JS, Saeidifar M, Ghasemi A, Ghaemi H, Heydari A (2017) Synthesis, characterization and in vitro antimicrobial screening of the xanthate derivates and their iron(II) complexes. Iran J Chem Chem Eng 36:43–54. https://doi.org/10.30492/IJCCE.2017.25477
Acknowledgements
The authors acknowledge the financial support received from the Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação (Proppi-UFF), and Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq). Daniella. B. Miranda acknowledges the doctorate fellowship granted for Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES). We would also like to thank the Laboratory of Computational Chemistry, LMQC – UFF.
Funding
This work was supported by Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ (Grant numbers E-26/210.302/2019 and E-26/211.091/2019), Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação-Proppi-UFF (FOPESQ-2020) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq) (Grant number 477833/2013–6).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design of this manuscript. Daniella Miranda wrote the first draft of the manuscript, and Dra. Susana Quintal and Dr. Glaucio Ferreira performed the critical review. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Miranda, D.B., Quintal, S. & Ferreira, G.B. Electronic analysis of n-propyl xanthate complexes with group 12 metals: a theoretical–experimental study. J Mol Model 30, 163 (2024). https://doi.org/10.1007/s00894-024-05950-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05950-z