Abstract
Context
Herein, we compare and contrast the dual roles of Cun clusters (n = 3, 5, and 7 atoms) in scavenging or generating RO• free radicals from ROH at the theoretical levels (where R = H, methyl, ethyl, n-propyl, i-propyl, n-butyl, t-butyl, and phenyl). This investigation is performed in water media to mimic the actual environment in the biological system. In the presence of the Cun clusters, bond dissociation energy (BDE) of RO–H and R–OH is reduced. This is clear evidence for the increased possibility of both the RO–H and R–OH bonds breakage and scavenging of RO• radicals. The nature of anchoring bonds responsible for the interaction of Cun clusters with ROH and RO• are interpreted using the quantum theory of atoms in molecules (QTAIM) and the natural bond orbital (NBO) analysis. The DFT results indicate that the O•⋅⋅⋅•Cu bond is stronger and has more covalent character in RO•⋅⋅⋅•Cun radical complexes than in ROH⋅⋅⋅•Cun. Therefore, the interactions of Cun clusters with RO• radicals (antioxidant) are more pronounced than their interactions with ROH non-radicals (pro-oxidant).
Methods
The GAMESS software package was utilized in this paper. The B3LYP and M06 functions with the 6–311 + + G(d,p), and LANL2DZ/SDD basis sets was used to perform the important geometrical parameters of RO•⋅⋅⋅•Cun and ROH⋅⋅⋅•Cun, binding energy (Eb), and bond dissociation energy (BDE).
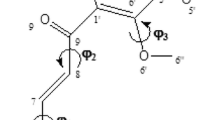
Graphical Abstract







Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Luo Z, Castleman A Jr, Khanna SN (2016) Chem Rev 116:14456–14492
Dietrich G, Krückeberg S, Lützenkirchen K, Schweikhard L, Walther C (2000) J Chem Phys 112:752–760
Spasov VA, Lee T-H, Ervin KM (2000) J Chem Phys 112:1713–1720
Mondal K, Manna D, Ghanty TK, Banerjee A (2014) Chem Phys 428:75–81
Mills G, Gordon MS, Metiu H (2002) Chem Phys Lett 359:493–499
Kryachko ES, Remacle F (2007) The gold-ammonia bonding patterns of neutral and charged complexes Au m±1–(NH3)n. I. Bonding and charge alternation. J Chem Phys 127:19
Wells DH Jr, Delgass WN, Thomson KT (2002) J Chem Phys 117:10597–10603
Lang SM, Bernhardt TM (2009) Cooperative and competitive coadsorption of H2, O2, and N2 on Aux+ (x= 3, 5). J Chem Phys 131(2)
Knickelbein MB (1992) Chem Phys Lett 192:129–134
Calaminici EP, Köster A, Russo N, Salahub D (1996) J Chem Phys 105:9546–9556
Katakuse I, Ichihara T, Fujita Y, Matsuo T, Sakurai T, Matsuda H (1985) Int J Mass Spectrom Ion Processes 67:229–236
Kimble M, Moore N, Castleman A, Bürgel C, Mitrić R, Bonačić-Koutecký V (2007) Eur Phys J D 43:205–208
Ma J, Cao X, Xing X, Wang X, Parks JH (2016) Phys Chem Chem Phys 18:743–748
Hagen J, Socaciu LD, Le Roux J, Popolan D, Bernhardt TM, Wöste L, Mitrić R, Noack H, Bonačić-Koutecký V (2004) J Am Chem Soc 126:3442–3443
Parks E, Nieman G, Kerns K, Riley S (1998) J Chem Phys 108:3731–3739
Kim YD, Stolcic D, Fischer M, Ganteför G (2003) J Chem Phys 119:10307–10312
Commoner B, Townsend J, Pake GE (1954) Nature 174:689–691
Alfadda AA, Sallam RM (20112) Reactive oxygen species in health and disease. Biomed Res Int 2012
Dhawan V (2014) Reactive oxygen and nitrogen species: general considerations. Studies on respiratory disorders. Springer, New York, pp 27–47
Liou G-Y, Storz P (2010) Free Radic Res 44:479–496
Hertog MG, Feskens EJ, Kromhout D, Hollman P, Katan M (1993) Lancet 342:1007–1011
Merry P, Winyard P, Morris C, Grootveld M, Blake D (1989) Ann Rheum Dis 48:864
Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues J-F (2000) Eur J Epidemiol 16:357–363
Boots AW, Li H, Schins RP, Duffin R, Heemskerk JW, Bast A, Haenen GR (2007) Toxicol Appl Pharmacol 222:89–96
Saller R, Meier R, Brignoli R (2001) Drugs 61:2035–2063
Gažák R, Marhol P, Purchartová K, Monti D, Biedermann D, Riva S, Cvak L, Křen V (2010) Process Biochem 45:1657–1663
Meister A (1995) Methods Enzymol 251:3–7
Rice-Evans CA, Miller NJ, Paganga G (1996) Free Radic Biol Med 20:933–956
Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Berghe DV (1998) J Nat Prod 61:71–76
Francisco-Marquez M, Galano A, Martínez A (2010) J Phys Chem C 114:6363–6370
Martínez A, Galano A (2010) J Phys Chem C 114:8184–8191
Marković Z, Milenković D, Đorović J, Marković JMD, Stepanić V, Lučić B, Amić D (2012) Food Chem 134:1754–1760
Çakmak E, ÖzbakırIşın D (2020) J Mol Model 26:1–11
Boulebd H, Khodja IA (2021) Phytochemistry 189:112831
Sun Y-M, Zhang H-Y, Chen D-Z, Liu C-B (2002) Org Lett 4:2909–2911
Kalaivanan C, Sankarganesh M, Suvaikin MY, Karthi GB, Gurusamy S, Subramanian R, Asha RN (2020) J Mol Liq 320:114423
Puškárová I, Breza M (2017) Chem Phys Lett 680:78–82
Damena T, Alem MB, Zeleke D, Desalegn T, Eswaramoorthy R, Demissie TB (2022) ACS Omega 7:26336–26352
Andrade E-B, Martínez A (2017) Comput Theor Chem 1115:127–135
Reina M, Martínez A (2017) Comput Theor Chem 1099:174–184
Reina M, Martínez A (2017) Comput Theor Chem 1112:1–9
Reina M, Martínez A (2017) Comput Theor Chem 1120:24–33
Ahmadi A, Kassaee MZ, Fattahi A (2018) J Phys Org Chem 31:e3776
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) J Comput Chem 14:1347–1363
Martínez A (2010) J Phys Chem C 114:21240–21246
Jug K, Zimmermann B, Calaminici P, Köster AM (2002) J Chem Phys 116:4497–4507
Fournier R (2001) J Chem Phys 115:2165–2177
Rezaee N, Ahmadi A, Kassaee MZ (2016) RSC Adv 6:13224–13233
Zheng D, Zhang M, Zhao G (2017) Sci Rep 7:1–10
Raghavachari K (2000) Theor Chem Acc 103:361–363
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206
Bouchareb F, Berredjem M, Dehmchi DA, Kadri R, Kadri M, Ferkous H, Mansouri A, Bouyegh S, Ahmed SA, Hadda TB (2023) J Mol Struct 1294:136503
Tedjeuguim CT, Tasheh SN, Alongamo CIL, Ghogomu JN (2022) J Chem Sci 134:70
Kosar N, Ayub K, Gilani MA, Muhammad S, Mahmood T (2022) ACS Omega 7:20800–20808
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Wadt WR, Hay PJ (1985) J Chem Phys 82:284–298
Weinhold F, Landis CR (2001) Chem Educ Res Pract 2:91–104
Weinhold F (2012) J Comput Chem 33:2363–2379
Glendening E, Landis C, Weinhold F (2012) Rev: Comput Mol Sci 2(10):1002
Weinhold F (1997) J Mol Struct: THEOCHEM 398:181–197
Bader RF (1991) Chem Rev 91:893–928
Lu T, Chen F (2012) J Comput Chem 33:580–592
Lu T, Chen F (2012) J Mol Graph Model 38:314–323
Scrocco E, Tomasi J (2005) The electrostatic molecular potential as a tool for the interpretation of molecular properties. New concepts II. Springer, Berlin, Heidelberg, pp 95–170
Bader RF, Nguyen-Dang T (1981) In advances in quantum chemistry. Elsevier 14:63–124
Espinosa E, Alkorta I, Elguero J, Molins E (2002) J Chem Phys 117:5529–5542
Lu F, Chen Y, Fu B, Chen S, Wang L (2022) Chin Chem Lett 33:5111–5115
Jacobsen H (2008) Can J Chem 86:695–702
Acknowledgements
The support from Tarbiat Modares University (TMU) is gratefully acknowledged.
Funding
Tarbiat Modares University.
Author information
Authors and Affiliations
Contributions
Batoul Alipour wrote the main manuscript text and has done the calculations, Tables, etc. Mohamad Zaman Kassaee is the corresponding author and wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alipour, B., Kassaee, M.Z. Comparison of Cu3, Cu5, and Cu7 clusters as potential antioxidants: A theoretical quest. J Mol Model 30, 132 (2024). https://doi.org/10.1007/s00894-024-05933-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05933-0