Abstract
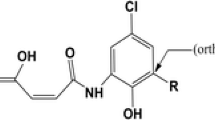
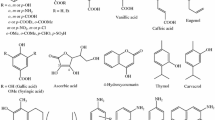
Chromone (4H-chromen-4-one, 4H-1-benzopyran-4-one) and related compounds are important pharmacophores and privileged structures in medicinal chemistry because of their important biological activities such as anti-tumor, anti-HIV, and antioxidant. In the study, the density functional theory (DFT) calculations were performed for radical scavenging activity evaluation of a series of 3-styrylchromone derivatives. The reaction enthalpies related to the steps in the radical scavenging action mechanisms and several physicochemical descriptors such as global hardness, softness, and electronegativity were computed in gas phase and in water. The solvation effect of water on the antioxidant activity was taken into account by using the conductor-like polarizable continuum model. The calculated results were discussed by considering all physicochemical properties of molecules: thermodynamic, orbital, and structural. The results obtained were consistent with the experimental results.



Similar content being viewed by others
References
Valko M, Rhodes C, Moncol J, Izakovic MM, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Panasenko OM, Vol’nova TV, Azizova OA, Vladimirov YA (1991) Free radical modification of lipoproteins and cholesterol accumulation in cells upon atherosclerosis. Free Radic Biol Med 10:137–148
Mokini Z, Marcovecchio ML, Chiarelli F (2010) Molecular pathology of oxidative stress in diabetic angiopathy: role of mitochondrial and cellular pathways. Diabetes Res Clin Pract 87:313–321
Butterfield DA (1997) β-Amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Chem Res Toxicol 10:495–506
Borges Bubols G, da Rocha VD, Medina-Remon A, von Poser G, Maria Lamuela-Raventos R, Lucia Eifler-Lima V, Cristina Garcia S (2013) The antioxidant activity of coumarins and flavonoids. Mini-Rev Med Chem 13:318–334
Shaw AY, Chang CY, Liau HH, Lu PJ, Chen HL, Yang CN, Li HY (2009) Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. Eur J Med Chem 44:2552–2562
Liu HS, Xu SQ, Cheng M, Chen Y, Xia P, Qian K, Lee KH (2011) Anti-AIDS agents 87. New bio-isosteric dicamphanoyl-dihydropyranochromone (DCP) and dicamphanoyl-khellactone (DCK) analogues with potent anti-HIV activity. Bioorg Med Chem Lett 21:5831–5834
Rao VM, Damu GLV, Sudhakar D, Siddaiah V, Rao CV (2008) New efficient synthesis and bioactivity of homoisoflavonoids. Arkivoc 11:285–294
Randhavane P, Karale B (2009) Synthesis and biological screening of some fluorinated dibenzofuran containing 3-chlorochromones and benzothiazepines. J Heterocyclic Chem 46:732–736
Nawrot-Modranka J, Nawrot E, Graczyk J (2006) In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur J Med Chem 41:1301–1309
Keri RS, Budagumpi S, Pai RK, Balakrishna RG (2014) Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem 78:340–374
Conti C, Desideri N (2010) New 4H-chromen-4-one and 2H-chromene derivatives as anti-picornavirus capsid-binders. Bioorg Med Chem 18:6480–6488
Sonawane SA, Chavan VP, Shingare MS, Karale BK (2002) Synthesis of 3-methyl-4-[(1, 3-diphenyl-1H-pyrazol-4-yl) methylene]-1-phenyl-pyrazolin-5 (4H)-ones and some 3-styrylchromones. Ind J Heterocycl Chem 12:65–66
Shimada C, Uesawa Y, Ishii-Nozawa R, Ishihara M, Kagaya H, Kanamoto T, Sakagami H (2014) Quantitative structure–cytotoxicity relationship of 3-styrylchromones. Anticancer Res 34:5405–5411
Takao K, Ishikawa R, Sugita Y (2014) Synthesis and biological evaluation of 3-styrylchromone derivatives as free radical scavengers and α-glucosidase inhibitors. Chem Pharm Bull 62:810–815
Mazzone G, Malaj N, Russo N, Toscano M (2013) Density functional study of the antioxidant activity of some recently synthesized resveratrol analogues. Food Chem 141:2017–2024
Xue Y, Zheng Y, An L, Dou Y, Liu Y (2014) Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem 151:198–206
Cai W, Chen Y, Xie L, Zhang H, Hou C (2014) Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur Food Res Technol 238:121–128
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01. Gaussian, Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO, version 5.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Cances E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041
Szeląg M, Mikulski D, Molski M (2012) Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J Mol Model 18:2907–2916
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Klein E, Rimarcik J, LukesV (2009) DFT/B3LYP study of the O–H bond dissociation enthalpies and proton affinities of para-and meta-substituted phenols in water and benzene. Acta Chim Slov 2:37–51
Rimarčík J, Lukeš V, Klein E, Ilčin M (2010) Study of the solvent effect on the enthalpies of homolytic and heterolytic N–H bond cleavage in p-phenylenediamine and tetracyano-p-phenylenediamine. J Mol Struct THEOCHEM 952:25–30
Parker VD (1992) Homolytic bond (HA) dissociation free energies in solution. Applications of the standard potential of the (H+/H. bul.) couple. J Am Chem Soc 114:7458–7462
Bizarro MM, Cabral BC, Dos Santos RB, Simões JM (1999) Substituent effects on the O–H bond dissociation enthalpies in phenolic compounds: agreements and controversies+ erratum. Pure Appl Chem 71:1249–1256
Janak JF (1978) Proof that∂ e∂ n i= ε in density-functional theory. Phys Rev B 18:7165
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Berghe DV (1998) Structure− activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 61:71–76
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Leopoldini M, Russo N, Toscano M (2006) Gas and liquid phase acidity of natural antioxidants. J Agric Food Chem 54:3078–3085
Özbakır ID (2016) Theoretical study on the investigation of antioxidant properties of some hydroxyanthraquinones. Mol Phys 114:3578–3588
Acknowledgments
This research was made possible by TUBITAK ULAKBIM, High Performance and Grid Computing Center (TR-Grid e-Infrastructure).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çakmak, E., Özbakır Işın, D. A theoretical evaluation on free radical scavenging activity of 3-styrylchromone derivatives: the DFT study. J Mol Model 26, 98 (2020). https://doi.org/10.1007/s00894-020-04368-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04368-7