Abstract
Context
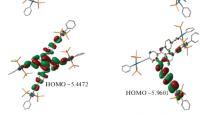
Schiff base-containing metal complexes have been the subject of extensive research. In this work, a coordination polymer-derived complex called [Cu(L)] that is solution-stable (L = 2-(2-hydroxybenzylidene-amino)phenol) has been explored theoretically with five different pyridyl-based ligands using DFT/TDDFT in order to understand the structural–functional and electronic transitions of these five complexes. Frontier molecular orbital (FMO) analysis was carried out to assess the reactivity behavior of all five complexes. For the purpose of studying the charge energy distribution over complexes, electrostatic potential maps were also drawn. Furthermore, in order to identify any stabilizing interactions that may be present in the given complexes, an NBO analysis was studied. To learn more about any potential correlations between the properties of these five complexes, a comparative analysis was explored. Our calculations demonstrate that complex 3 having pyridine-4-carboxamide as a ligand has a lower energy gap and a higher negative electrostatic potential which may indicate its higher reactivity and this may be due to the electron withdrawing group (carboxamide). TDDFT results show that the highest light harvesting efficiency (LHE) of all the studied complexes is found in the range of 440–448 nm. Complexes 1, 2, and 4 show the higher light harvesting efficiency as compared to complexes 3 and 5. Our findings are in good accordance with the available experimental data.
Methods
All DFT computations were performed using the Gaussian16 with unrestricted B3LYP-D2 functional with the basis sets 6-31G(d,p) for O, N, C, and H while LanL2DZ for Cu. The polarized continuum model (PCM) was used for the solvation. The software GaussView6.1 was utilized for the modeling of initial geometries and the plotting of MEP maps. The NBO6.0 program which is incorporated in Gaussian16 was utilized to investigate the bonding nature and stabilization energies of the complexes. The ORCA program was used to simulate the absorption spectra.








Similar content being viewed by others
Data availability
Data available within the manuscript and its supplementary materials file.
References
Naqi Ahamad M, Iman K, Raza MK et al (2020) Anticancer properties, apoptosis and catecholase mimic activities of dinuclear cobalt(II) and copper(II) Schiff base complexes. Bioorg Chem 95:103561. https://doi.org/10.1016/j.bioorg.2019.103561
Mantasha I, Shahid M, Kumar M et al (2020) Exploring solvent dependent catecholase activity in transition metal complexes: an experimental and theoretical approach. New J Chem 44:1371–1388. https://doi.org/10.1039/C9NJ04374H
Keypour H, Rezaeivala M, Valencia L et al (2009) Synthesis and characterization of some new Co(II) and Cd(II) macroacyclic Schiff-base complexes containing piperazine moiety. Polyhedron 28:3755–3758. https://doi.org/10.1016/j.poly.2009.08.021
Xing A, Zeng D, Chen Z (2022) Synthesis, crystal structure and antioxidant activity of butylphenol Schiff bases: experimental and DFT study. J Mol Struct 1253:132209. https://doi.org/10.1016/j.molstruc.2021.132209
Bhalla P, Tomer N, Goel A et al (2022) Chemoselective detection based on experimental and theoretical calculations of Cu2+ ions via deprotonation of chromone derived probe and its application. J Mol Struct 1264:133251. https://doi.org/10.1016/j.molstruc.2022.133251
Shahid M, Mantasha I, Khan S et al (2021) Elucidating the contribution of solvent on the catecholase activity in a mononuclear Cu(II) system: an experimental and theoretical approach. J Mol Struct 1244:130878. https://doi.org/10.1016/j.molstruc.2021.130878
Tisato F, Refosco F, Bandoli G (1994) Structural survey of technetium complexes. Coord Chem Rev 135–136:325–397. https://doi.org/10.1016/0010-8545(94)80072-3
Kitaura R, Onoyama G, Sakamoto H et al (2004) Immobilization of a metallo Schiff base into a microporous coordination polymer. Angew Chemie Int Ed 116:2738–2741. https://doi.org/10.1002/ange.200352596
Shultz AM, Sarjeant AA, Farha OK et al (2011) Post-synthesis modification of a metal–organic framework to form metallosalen-containing MOF materials. J Am Chem Soc 133:13252–13255. https://doi.org/10.1021/ja204820d
Vardhan H, Yusubov M, Verpoort F (2016) Self-assembled metal–organic polyhedra: an overview of various applications. Coord Chem Rev 306:171–194. https://doi.org/10.1016/j.ccr.2015.05.016
Tranchemontagne DJ, Mendoza-Cortés JL, O’Keeffe M, Yaghi OM (2009) Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem Soc Rev 38:1257. https://doi.org/10.1039/b817735j
Yaghi OM, O’Keeffe M, Ockwig NW et al (2003) Reticular synthesis and the design of new materials. Nature 423:705–714. https://doi.org/10.1038/nature01650
Mullaney BR, Goux-Capes L, Price DJ et al (2017) Spin crossover-induced colossal positive and negative thermal expansion in a nanoporous coordination framework material. Nat Commun 8:1053. https://doi.org/10.1038/s41467-017-00776-1
Elgrishi N, Chambers MB, Wang X, Fontecave M (2017) Molecular polypyridine-based metal complexes as catalysts for the reduction of CO2. Chem Soc Rev 46:761–796. https://doi.org/10.1039/C5CS00391A
Anastasiadis NC, Polyzou CD, Kostakis GE et al (2015) Dinuclear lanthanide(III)/zinc(II) complexes with methyl 2-pyridyl ketone oxime. Dalton Trans 44:19791–19795. https://doi.org/10.1039/C5DT03663A
Mulrooney DZT, Clements JE, Ericsson DJ et al (2018) Phase control of ferromagnetic copper(II) carbonate coordination polymers through reagent concentration. Eur J Inorg Chem 2018:5223–5228. https://doi.org/10.1002/ejic.201801041
Maza WA, Haring AJ, Ahrenholtz SR et al (2016) Ruthenium(II)-polypyridyl zirconium(IV) metal–organic frameworks as a new class of sensitized solar cells. Chem Sci 7:719–727. https://doi.org/10.1039/C5SC01565K
Poynton FE, Bright SA, Blasco S et al (2017) The development of ruthenium(II) polypyridyl complexes and conjugates for in vitro cellular and in vivo applications. Chem Soc Rev 46:7706–7756. https://doi.org/10.1039/C7CS00680B
Cardin CJ, Kelly JM, Quinn SJ (2017) Photochemically active DNA-intercalating ruthenium and related complexes–insights by combining crystallography and transient spectroscopy. Chem Sci 8:4705–4723. https://doi.org/10.1039/C7SC01070B
Beddoe SVF, Fitzpatrick AJ, Price JR et al (2017) A bridge too far: testing the limits of polypyridyl ligands in bridging soluble subunits of a coordination polymer. Cryst Growth Des 17:6603–6612. https://doi.org/10.1021/acs.cgd.7b01256
Beddoe SVF, Lonergan RF, Pitak MB et al (2019) All about that base: investigating the role of ligand basicity in pyridyl complexes derived from a copper-Schiff base coordination polymer. Dalton Trans 48:15553–15559. https://doi.org/10.1039/C9DT01527B
Koley MK, Parsekar SU, Duraipandy N et al (2018) DNA binding and cytotoxicity of two Cu(II) complexes containing a Schiff base ligand along with 1,10-phenanthroline or imidazole as a coligand. Inorganica Chim Acta 478:211–221. https://doi.org/10.1016/j.ica.2018.04.017
Abdel-Rahman LH, Basha MT, Al-Farhan BS et al (2023) Synthesis, Characterization, DFT studies of novel Cu(II), Zn(II), VO(II), Cr(III), and La(III) chloro-substituted Schiff base complexes: aspects of its antimicrobial, antioxidant, anti-inflammatory, and photodegradation of methylene blue. Molecules 28:4777–4777. https://doi.org/10.3390/molecules28124777
Yusuf Tunde L, Oladipo SD, Zamisa S et al (2021) Design of new Schiff-base copper(II) complexes: synthesis, crystal structures, DFT study, and binding potency toward cytochrome P450 3A4. ACS Omega 6:13704–13718. https://doi.org/10.1021/acsomega.1c00906
Vishwakarma PK, Mir JM, Maurya RC (2016) Pyrone-based Cu(II) complexes, their characterization, DFT based conformational drift from square planar to square pyramidal geometry and biological activities. J Chem Sci 128:511–522. https://doi.org/10.1007/s12039-016-1048-6
Sutradhar M, Alegria A, Tannistha RB et al (2020) 1D Copper(II)-aroylhydrazone coordination polymers: magnetic properties and microwave assisted oxidation of a secondary alcohol. Front Chem 8:157–157. https://doi.org/10.3389/fchem.2020.00157
Chavez-Urias IF, López-González LE, Plascencia-Martínez DF et al (2023) l-Isoleucine-Schiff base copper(II) coordination polymers: crystal structure, spectroscopic, hirshfeld surface, and dft analyses. ACS Omega 8:24601–24614. https://doi.org/10.1021/acsomega.3c02878
Frisch MJ, Trucks GW, Schlegel HB, et al (2016) Gaussian16, Wallingford, CT. Gaussian16 (Revision A.03).
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Gray A, Tsybizova A, Roithova J (2015) Carboxylate-assisted C-H activation of phenylpyridines with copper, palladium and ruthenium: a mass spectrometry and DFT study. Chem Sci 6:5544–5553. https://doi.org/10.1039/c5sc01729g
Huang WK, Chen WT, Hsu IJ et al (2017) Cross C-S coupling reaction catalyzed by copper(I) N-heterocyclic carbene complexes. RSC Adv 7:4912–4920. https://doi.org/10.1039/c6ra27757h
Alshammari N, Platts JA (2020) Theoretical study of copper binding to GHK peptide. Comput Biol Chem 86:107265. https://doi.org/10.1016/j.compbiolchem.2020.107265
Lonsdale R, Harvey JN, Mulholland AJ (2012) Effects of dispersion in density functional based quantum mechanical/molecular mechanical calculations on cytochrome p450 catalyzed reactions. J Chem Theory Comput 8:4637–4645. https://doi.org/10.1021/ct300329h
Ansari A, Kaushik A, Rajaraman G (2013) Mechanistic insights on the ortho -hydroxylation of aromatic compounds by non-heme iron complex: a computational case study on the comparative oxidative ability of ferric-hydroperoxo and high-valent FeIV═O and FeV═O intermediates. J Am Chem Soc 135:4235–4249. https://doi.org/10.1021/ja307077f
Ansari A, Rajaraman G (2014) ortho-Hydroxylation of aromatic acids by a non-heme FeV=O species: how important is the ligand design? Phys Chem Chem Phys 16:14601–14613. https://doi.org/10.1039/C3CP55430A
Jayapal P, Ansari A, Rajaraman G (2015) Computational examination on the active site structure of a (peroxo) diiron (III) intermediate in the amine oxygenase AurF. Inorg Chem 54:11077–11082. https://doi.org/10.1021/acs.inorgchem.5b00872
Kepp KP (2011) The ground states of iron (III) porphines: role of entropy–enthalpy compensation, Fermi correlation, dispersion, and zero-point energies. J Inorg Biochem 105:1286–1292. https://doi.org/10.1016/j.jinorgbio.2011.07.012
Yadav O, Kumar M, Mittal H et al (2022) Theoretical exploration on structures, bonding aspects and molecular docking of α-aminophosphonate ligated copper complexes against SARS-CoV-2 proteases. Front pharmacol 13:982484. https://doi.org/10.3389/fphar.2022.982484
Da Silva JCS, Pennifold RCR, Harvey JN, Rocha WR (2016) A radical rebound mechanism for the methane oxidation reaction promoted by the dicopper center of a pMMO enzyme: a computational perspective. Dalton Trans 45:2492–2504. https://doi.org/10.1039/c5dt02638e
Jangir R, Ansari M, Kaleeswaran D et al (2019) Unprecedented copper (II) complex with a topoquinone-like moiety as a structural and functional mimic for copper amine oxidase: role of copper(II) in the genesis and amine oxidase activity. ACS Catal 9:10940–10950. https://doi.org/10.1021/acscatal.9b02326
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728. https://doi.org/10.1063/1.1674902
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283. https://doi.org/10.1063/1.448799
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298. https://doi.org/10.1063/1.448800
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310. https://doi.org/10.1063/1.448975
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297. https://doi.org/10.1039/b508541a
Chocholoušová J, Špirko V, Hobza P (2004) First local minimum of the formic acid dimer exhibits simultaneously red-shifted O-H⋯O and improper blue-shifted C–H⋯O hydrogen bonds. Phys Chem Chem Phys 6:37–41. https://doi.org/10.1039/B314148A
van Wüllen C (1998) Molecular density functional calculations in the regular relativistic approximation: method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. Chem Phys 109:392–399. https://doi.org/10.1063/1.476576
van Lenthe E, Baerends EJ, Snijders JG (1993) Relativistic regular two-component Hamiltonians. Phys Rev B 99:4597–4610. https://doi.org/10.1063/1.466059
van Lenthe E, Baerends EJ, Snijders JG (1994) Relativistic total energy using regular approximations. Phys Rev B 101:9783–9792. https://doi.org/10.1063/1.467943
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/physrevb.37.785
Becke AD (1992) Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J Chem Phys 96:2155–2160. https://doi.org/10.1063/1.462066
Neese F (2018) Software update: the ORCA program system, version 4.0. WIREs Comput Mol Sci 8:e1327. https://doi.org/10.1002/wcms.1327
Yadav O, Ansari M, Ansari A (2022) Electronic structures, bonding aspects and spectroscopic parameters of homo/hetero valent bridged dinuclear transition metal complexes. Spectrochim Acta A Mol Biomol Spectrosc 278:121331. https://doi.org/10.1016/j.saa.2022.121331
Kumar M, Ansari M, Ansari A (2023) Electronic, geometrical and photophysical facets of five coordinated porphyrin N-heterocyclic carbene transition metals complexes: a theoretical study. Spectrochim Acta A Mol Biomol Spectrosc 284:121774. https://doi.org/10.1016/j.saa.2022.121774
Sahu R, Mohapatra RK, Al-Resayes SI et al (2021) An efficient synthesis towards the core of Crinipellin: TD-DFT and docking studies. J Saudi Chem Soc 25:101193. https://doi.org/10.1016/j.jscs.2020.101193
Irfan A, Al-Sehemi AG (2012) Quantum chemical study in the direction to design efficient donor-bridge-acceptor triphenylamine sensitizers with improved electron injection. J Mol Model 18:4893–4900. https://doi.org/10.1007/s00894-012-1488-y
Kumar M, Gupta MK, Rizvi MA, Ansari A (2023) Electronic structures and ligand effect on redox potential of iron and cobalt complexes: a computational insight. Struct Chem 34:1565–1575. https://doi.org/10.1007/s11224-022-02119-3
Monika AA (2023) Electronic structures and energetic of metal(II)-superoxo species: a DFT exploration. Struct Chem 34:825–835. https://doi.org/10.1007/s11224-022-02030-x
Lawal MM, Govender T, Maguire GEM et al (2016) Mechanistic investigation of the uncatalyzed esterification reaction of acetic acid and acid halides with methanol: a DFT study. J Mol Model 22:235. https://doi.org/10.1007/s00894-016-3084-z
Kerru N, Gummidi L, Bhaskaruni SVHS et al (2019) A comparison between observed and DFT calculations on structure of 5-(4-chlorophenyl)-2-amino-1,3,4-thiadiazole. Sci Rep 9:19280. https://doi.org/10.1038/s41598-019-55793-5
Monika YO, Chauhan H, Ansari A (2021) Electronic structures, bonding, and spin state energetics of biomimetic mononuclear and bridged dinuclear iron complexes: a computational examination. Struct Chem 32:1473–1488. https://doi.org/10.1007/s11224-020-01690-x
Drissi M, Benhalima N, Megrouss Y et al (2015) Theoretical and experimental electrostatic potential around the m-nitrophenol molecule. Molecules 20:4042–4054. https://doi.org/10.3390/molecules20034042
Sumrra SH, Atif AH, Zafar MN et al (2018) Synthesis, crystal structure, spectral and DFT studies of potent isatin derived metal complexes. J Mol Struct 1166:110–120. https://doi.org/10.1016/j.molstruc.2018.03.132
Yadav O, Ansari M, Ansari A (2021) Electronic structures, bonding and energetics of non-heme mono and dinuclear iron-TPA complexes: a computational exploration. Struct Chem 32:2007–2018. https://doi.org/10.1007/s11224-021-01775-1
Tanak H, Koysal Y, Isik S et al (2011) Experimental and computational approaches to the molecular structure of 3-(2-mercaptopyridine)phthalonitrile. Bull Korean Chem Soc 32:673–680. https://doi.org/10.5012/bkcs.2011.32.2.673
Acknowledgements
AA would like to thank the Central University of Haryana for providing computing facilities.
Funding
MA would like to thank the Central University of Haryana for its financial support.
Author information
Authors and Affiliations
Contributions
Mukhtar Ahmed: calculations, validation, visualization, writing—original draft; Manoj Kumar Gupta: editing; Azaj Ansari: supervised this research.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors provided consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, M., Gupta, M.K. & Ansari, A. DFT and TDDFT exploration on the role of pyridyl ligands with copper toward bonding aspects and light harvesting. J Mol Model 29, 358 (2023). https://doi.org/10.1007/s00894-023-05765-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05765-4