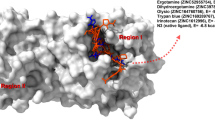
Abstract
Context
Modulation of disease progression is frequently started by identifying biochemical pathway catalyzed by biomolecule that is prone to inhibition by small molecular weight ligands. Such ligands (leads) can be obtained from natural resources or synthetic libraries. However, de novo design based on fragments assembly and optimization is showing increasing success. Plasmodium falciparum parasite depends on glutathione-S-transferase (PfGST) in buffering oxidative heme as an approach to resist some antimalarials. Therefore, PfGST is considered an attractive target for drug development. In this research, fragment-based approaches were used to design molecules that can fit to glutathione (GSH) binding site (G-site) of PfGST.
Methods
The involved approaches build molecules from fragments that are either isosteric to GSH sub-moieties (ligand-based) or successfully docked to GSH binding sub-pockets (structure-based). Compared to reference GST inhibitor of S-hexyl GSH, ligands with improved rigidity, synthetic accessibility, and affinity to receptor were successfully designed. The method involves joining fragments to create ligands. The ligands were then explored using molecular docking, Cartesian coordinate’s optimization, and simplified free energy determination as well as MD simulation and MMPBSA calculations. Several tools were used which include OPENEYE toolkit, Open Babel, Autodock Vina, Gromacs, and SwissParam server, and molecular mechanics force field of MMFF94 for optimization and CHARMM27 for MD simulation. In addition, in-house scripts written in Matlab were used to control fragments connection and automation of the tools.












Similar content being viewed by others
References
Wang S, Dong G, Sheng C (2019) Structural simplification of natural products. Chem Rev 119(6):4180–4220
de Souza Neto LR et al (2020) In silico strategies to support fragment-to-lead optimization in drug discovery. Front Chem 8(93). https://doi.org/10.3389/fchem.2020.00093
Mouchlis VD et al (2021) Advances in de novo drug design: from conventional to machine learning methods. Int J Mol Sci 22(4). https://doi.org/10.3390/ijms22041676
Devi R, Sathya S, Coumar M (2015) Evolutionary algorithms for de novo drug design – a survey. Appl Soft Comput 27:543–552
Devereux M, LA Popelier P (2010) In silico techniques for the identification of bioisosteric replacements for drug design. Curr Top Med Chem 10(6):657–668
Rachman M et al (2021) Fragment-to-lead tailored in silico design. Drug Discov Today Technol 40:44–57
Chu Y, He X (2019) MoleGear: a Java-based platform for evolutionary de novo molecular design. Molecules 24(7):1444
Spiegel JO, Durrant JD (2020) AutoGrow4: an open-source genetic algorithm for de novo drug design and lead optimization. J Cheminform 12(1):25
Kawai K, Nagata N, Takahashi Y (2014) De novo design of drug-like molecules by a fragment-based molecular evolutionary approach. J Chem Inf Model 54(1):49–56
Olivecrona M et al (2017) Molecular de-novo design through deep reinforcement learning. J Cheminform 9(1):48
Meyers J, Fabian B, Brown N (2021) De novo molecular design and generative models. Drug Discov Today 26(11):2707–2715
Sheng C, Zhang W (2013) Fragment informatics and computational fragment-based drug design: an overview and update. Med Res Rev 33(3):554–598
Bian Y, Xie X-QS (2018) Computational fragment-based drug design: current trends, strategies, and applications. AAPS J 20(3):59–59
Colón-Lorenzo EE et al (2020) Structure-based screening of Plasmodium berghei glutathione S-transferase identifies CB-27 as a novel antiplasmodial compound. Front Pharmacol 11(246). https://doi.org/10.3389/fphar.2020.00246
Hiller N et al (2006) Plasmodium falciparum glutathione S-transferase--structural and mechanistic studies on ligand binding and enzyme inhibition. Protein Sci 15(2):281–289
Al-Qattan MN, Mordi MN, Mansor SM (2016) Assembly of ligands interaction models for glutathione-S-transferases from Plasmodium falciparum, human and mouse using enzyme kinetics and molecular docking. Comput Biol Chem 64:237–249
Wlodek S, Skillman AG, Nicholls A (2010) Ligand entropy in gas-phase, upon solvation and protein complexation. Fast estimation with quasi-newton hessian. J Chem Theory Comput 6:2140–2152
Word JM, Lovell SC, Richardson JS, Richardson DC (1999) Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J Mol Biol 285:1735–1747
Sharp KA (2012) Statistical thermodynamics of binding and molecular recognition models. Protein-Ligand Interactions. Wiley-VCH Verlag GmbH & Co. KGaA, pp 1–22
Kuhn B et al (2003) MM-PBSA applied to computer-assisted ligand design. ChemInform 34(15). https://doi.org/10.1002/chin.200315293
Srinivasan J et al (1998) Continuum solvent studies of the stability of DNA, RNA, and phosphoramidate−DNA helices. J Am Chem Soc 120(37):9401–9409
Grant JA et al (2007) A simple formula for dielectric polarisation energies: the Sheffield solvation model. Chem Phys Lett 441(1–3):163–166
Hou T et al (2011) Assessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular mechanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from docking. J Comput Chem 32(5):866–877
Lyne PD, Lamb ML, Saeh JC (2006) Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoring. J Med Chem 49(16):4805–4808
Reddy MR, Erion MD (2001) Free energy calculations in rational drug design. Springer
O'Boyle NM et al (2011) Open Babel: an open chemical toolbox. J Cheminform 3(1):33
Zoete V et al (2011) SwissParam: a fast force field generation tool for small organic molecules. J Comput Chem 32(11):2359–2368
Kumari R, Kumar R, Lynn A (2014) g_mmpbsa—a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54(7):1951–1962
Armstrong RN (1997) Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol 10(1):2–18
Adang AE et al (1990) The glutathione-binding site in glutathione S-transferases. Investigation of the cysteinyl, glycyl and γ-glutamyl domains. Biochem J 269(1):47–54
Ruzza P et al (2009) Glutathione transferases as targets for cancer therapy. Anti Cancer Agents Med Chem 9(7):763–777
Liebau E et al (2005) Cooperativity and pseudo-cooperativity in the glutathione S-transferase from Plasmodium falciparum. J Biol Chem 280(28):26121–26128
Burg D et al (2002) Inhibition of glutathione S-transferase in rat hepatocytes by a glycine-tetrazole modified S-alkyl–GSH analogue. Bioorg Med Chem Lett 12(12):1579–1582
Cacciatore I et al (2005) Potent isozyme-selective inhibition of human glutathione S-transferase A1-1 by a novel glutathione S-conjugate. Amino Acids 29(3):255–261
Klotz P et al (1998) Synthesis and glutathione S-transferase structure-affinity relationships of nonpeptide and peptidase-stable glutathione analogues. J Med Chem 41(13):2278–2288
Boda K, Seidel T, Gasteiger J (2007) Structure and reaction based evaluation of synthetic accessibility. J Comput Aided Mol Des 21(6):311–325
Baker J (1993) Techniques for geometry optimization: a comparison of cartesian and natural internal coordinates. J Comput Chem 14(9):1085–1100
McGann M (2011) FRED pose prediction and virtual screening accuracy. J Chem Inf Model 51(3):578–596
Houston DR, Walkinshaw MD (2013) Consensus docking: improving the reliability of docking in a virtual screening context. J Chem Inf Model 53(2):384–390
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17(5-6):490–519
McGann M (2012) FRED and HYBRID docking performance on standardized datasets. J Comput Aided Mol Des 26(8):897–906
McGann M et al (2003) Gaussian docking functions. Biopolymers 68(1):76–90
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Boittier ED et al (2020) Assessing molecular docking tools to guide targeted drug discovery of CD38 inhibitors. Int J Mol Sci 21(15):5183
Kunze T (1996) Phosphono analogues of glutathione as new inhibitors of glutathione S-transferases. Arch Pharm 329(11):503–509
Liu K, Watanabe E, Kokubo H (2017) Exploring the stability of ligand binding modes to proteins by molecular dynamics simulations. J Comput Aided Mol Des 31(2):201–211
Guterres H, Im W (2020) Improving protein-ligand docking results with high-throughput molecular dynamics simulations. J Chem Inf Model 60(4):2189–2198
Shen X-L et al (2012) Computer-aided de novo ligand design and docking/molecular dynamics study of vitamin D receptor agonists. J Mol Model 18(1):203–212
Poli G et al (2020) Application of MM-PBSA methods in virtual screening. Molecules 25(8):1971
Homeyer N, Gohlke H (2012) Free energy calculations by the molecular mechanics Poisson−Boltzmann surface area method. Mol Inf 31(2):114–122
Gilson MK, Zhou HX (2007) Calculation of protein-ligand binding affinities. Annu Rev Biophys Biomol Struct 36:21–42
Stella L et al (1999) Molecular dynamics simulations of human glutathione transferase P1–1: conformational fluctuations of the apo-structure. Proteins Struct Funct Bioinf 37(1):10–19
Stella L et al (1999) Molecular dynamics simulations of human glutathione transferase P1-1: analysis of the induced-fit mechanism by GSH binding. Proteins Struct Funct Bioinf 37(1):1–9
Omae Y et al (2012) Molecular dynamics study of glutathione S-transferase: structure and binding character of glutathione. In: Nishikawa K et al (eds) Quantum Systems in Chemistry and Physics. Springer, Netherlands, pp 545–553
Harwaldt P, Rahlfs S, Becker K (2002) Glutathione S-transferase of the malarial parasite Plasmodium falciparum: characterization of a potential drug target. Biol Chem 383(5):821–830
Data availability
Available in supplementary materials. The scripts using in designing IFR fragments as well data analysis are available in supplementary material.
The full database of generated IFR and DFR fragments are available on request.
The Molinspiration fragments database were kindly provided by Dr. Macky Slimak as in attached email.
Dear Mohammed,
please find the Molinspiration collection of drug-like substituents and linkers, together with calculated properties, attached.
More details about calculated properties are available at http://www.molinspiration.com/services/properties.html.
By using the data, you agree that the data will be used purely for scientific / academic research (no industry collaborations) and will not be distributed outside your working group. We require also that Molinspiration is acknowledged in eventual presentations or publications using these data.
Be so kind and confirm, that you received the data safely.
Kind regards,
Dr. Macky Slimak
CTO, Molinspiration Cheminformatics
Code availability
Available in supplementary materials.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Dr. Mohammed Nooraldeen proposed the topic, handled research, and wrote manuscript while Prof Mohd Nizam Mordi provided consultations and manuscript reviewing.
Corresponding author
Ethics declarations
This work was supported by research fellowship program provided by Universiti Sains Malaysia. The authors have no relevant financial or non-financial interests to disclose. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Some of scripts used are avaliable on Github repository https://github.com/mohammednooraldeen.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 8877 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Qattan, M.N.M., Mordi, M.N. Development and application of fragment-based de novo inhibitor design approaches against Plasmodium falciparum GST. J Mol Model 29, 281 (2023). https://doi.org/10.1007/s00894-023-05650-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05650-0