Abstract
Context
Graphene has been used as reinforcement of polymeric nanocomposites to increase mechanical and electrical properties. Recently, graphene suspensions have been used for the development of nanofluids in automotive applications, where improvements in convection heat transfer coefficients and pressure drops have been reported. However, dispersions of graphene sheets in a polymeric matrix as well as in a solvent medium are difficult to achieve; that is because Van der Waals, \(\pi -\pi \) and Coulombic interactions cause agglomerations. Surface chemical modifications have been considered as viable options to improve the graphene integration. In this work, we studied the colloidal stability of aqueous solutions of graphene sheets functionalized with (i) carboxylic groups, (ii) 3-amino-propyl tri-ethoxy silane (amphiphilic behavior), (iii) graphene oxide, and (iv) pristine graphene. Results show that the lower sedimentation velocity corresponds to the graphene functionalized with carboxylic groups, which presents the higher colloidal stability. However, the amphiphilic group enhances the interaction energy between graphene and the solvent; we believe that there is a threshold percentage of functionalization that improves the colloidal stability of graphene.
Method
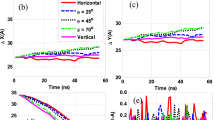
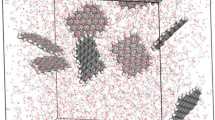
Transport properties of graphene solutions were estimated by using Non-Equilibrium Molecular Dynamics simulations to generate Poiseuille flow in an NVT ensemble. Simulations were developed in the LAMMPS code. The COMPASS Force Field was used for the graphene systems and the TIP3P for the water molecules. Bonds and angles of hydrogen atoms were kept rigid by using the shake algorithm. The molecular models were built through MedeA and visualized with the Ovito software.








Similar content being viewed by others
References
Perez EM, Martin N (2015) \(\pi - \pi \) interactions in carbon nanostructures. Chem Soc Rev 44:6425–6433
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50(9):3210–3228 (Festschrift dedicated to Peter A. Thrower, Editor-in-Chief, 1972 - 2012)
Janiak C (2000) A critical account on \(\pi -\pi \) stacking in metal complexes with aromatic nitrogen-containing ligands. J Chem Soc Dalton Trans, 3885–3896
Martinez CR, Iverson BL (2012) Rethinking the term “pi-stacking” Chem Sci 3:2191–2201
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS (2011) Graphene-based polymer nanocomposites. Polymer 52(1):5–25
Young RJ, Kinloch IA, Gong L, Novoselov KS (2012) The mechanics of graphene nanocomposites: A review. Compos Sci Technol 72(12):1459–1476
Morimoto N, Kubo T, and Nishina Y (2016) Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci Rep, 6
Krzysztof T, Lukasz M, Lukasz S, Blazej S (2018) Preparation and characterization of partially reduced graphene oxide aerogels doped with transition metal ions. J Mater Sci 53:16086–16098
Baglio V, Ahn M, Liu R, Lee C, Lee W (2019) Designing carbon/oxygen ratios of graphene oxide membranes for proton exchange membrane fuel cells. J Nanomater, 2019
Ahmed J, Tabish TA, Zhang S, Edirisinghe M (2021) Porous graphene composite polymer fibres. Polymers, 13(1)
Marsden AJ , Papageorgiou DG , Vallés C, Liscio A, Palermo V, Bissett MA , Young RJ, Kinloch IA (2018) Electrical percolation in graphene–polymer composites. 2D Mater 5(3):032003
Othman NH, Ismail MC, Mustapha M, Sallih N, Kee KE, Jaal RA (2019) Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog Org Coat 135:82–99
Selvam C, Mohan Lal D, Harish S (2017) Enhanced heat transfer performance of an automobile radiator with graphene based suspensions. Appl Therm Eng 123:50–60
Peng H, Jing S, Suyun T, Siwei Y, Shengju D, Guqiao D, Xiaoming X, Mianheng J (2015) Processable aqueous dispersions of graphene stabilized by graphene quantum dots. Chem Mater 27:218–226
Wang W, Zhang Y, Wang Y-B (2014) Noncovalent \(\pi \cdots \pi \) interaction between graphene and aromatic molecule: Structure, energy, and nature. J Chem Phys 140(9):094302
Bharathi K, Sukumaran V (2012) Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pka measurements. J Phys Chem Lett 3:867–872
McCoy TM, Turpin G, Teo BM, Tabor RF (2019) Graphene oxide: a surfactant or particle? Curr Opin Colloid Interface Sci 39:98–109 (Special Topic Section: Outstanding Young Researchers in Colloid and Interface Science)
León V, Rodriguez AM, Prieto P, Prato M, Vázquez E (2014) Exfoliation of graphite with triazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 8:563–571
Hsieh AG, Korkut S, Punckt C, Aksay IA (2013) Dispersion stability of functionalized graphene in aqueous sodium dodecyl sulfate solutions. Langmuir 29:14831–14838
Ling S, Wang Q, Zhang D, Zhang Y, Xuan M, Kaplan DL, Buehler MJ (2018) Integration of stiff graphene and tough silk for the design and fabrication of versatile electronic materials. Adv Funct Mater 28(9):1705291
Xiaozhen F, Xing L, Zhenglin H, Kaiyuan Z, Guosheng S (2022) Dft study of common anions adsorption at graphene surface due to anion-\(\pi \) interaction. J Mol Model 28(8):225
Rubio-Pereda P, Takeuchi N (2016) Van der waals molecular interactions in the organic functionalization of graphane, silicane, and germanane with alkene and alkyne molecules: a dft-d2 study. J Mol Model 22(8):175
Shih C-J, Lin S, Strano MS, Blankschtein D (2010) Understanding the stabilization of liquid-phase-exfoliated graphene in polar solvents: Molecular dynamics simulations and kinetic theory of colloid aggregation. J Am Chem Soc 132(41):14638–14648
Chen S, Li Q, He D, Liu Y, Wang L, Wang M (2022) Aggregation behavior of partially contacted graphene sheets in six-carbon alkanes: all-atom molecular dynamics simulation. J Mol Model 28(6):169
Kurban M, Erkoç Ş (2017) Segregation formation, thermal and electronic properties of ternary cubic cdznte clusters: Md simulations and dft calculations. Phys E Low Dimens Syst Nanostruc 88:243–251
Kurban M, Malcioğlu OB, Erkoç Ş (2016) Structural and thermal properties of cd-zn-te ternary nanoparticles: Molecular-dynamics simulations. Chem Phys 464:40–45
Matsubara H, Kikugawa G, Bessho T, Yamashita S, Ohara T (2016) Non-equilibrium molecular dynamics simulation as a method of calculating thermodynamic coefficients. Fluid Phase Equilibria 421:1–8
Castrejón-González O, Castillo-Tejas J, Manero O, Alvarado JFJ (2013) Structure factor and rheology of chain molecules from molecular dynamics. J Chem Phys 138(18):184901
Castrejón-González EO, Márquez Baños VE, Javier Alvarado JF, Rico-Ramírez Castillo-Tejas J, Jimíenez-Islas H (2014) Rheological model for micelles in solution from molecular dynamics. J Mol Liq 198:84–93
Castillo-Tejas J, Castrejón-González O, Carro S, González-Coronel V, Alvarado JFJ, Manero O (2016) Associative polymers. part iii: Shear rheology from molecular dynamics. Colloids Surf A: Physicochem Eng Asp 491:37–49
Blanco-Díaz EG, Castrejón-González EO, Javier Alvarado JF, Estrada-Baltazar A, Castillo-Borja F (2017) Rheological behavior of ionic liquids: analysis of the h-bond formation by molecular dynamics. J Mol Liq 242:265–271
Blanco-Díaz EG, Castrejón-González EO, Rico-Ramírez V, Aztatzi-Pluma D, Díaz-Ovalle CO (2018) Polydispersity influence in rheological behavior of linear chains by molecular dynamics. J Mol Liq 268:832–839
Macias-Jamaica RE, Castrejón-González EO, González-Alatorre G, Alvarado JFJ, Díaz-Ovalle CO (2019) Molecular models for sodium dodecyl sulphate in aqueous solution to reduce the micelle time formation in molecular simulation. J Mol Liq 274:90–97
Blanco-Díaz EG, González-Alatorre G, Lona-Ramírez FJ, Castrejón-González EO (2021) Effect of increasing the cation chain length on thermodynamic and transport properties of ionic liquids using molecular dynamics. J Mol Liq 334:116430
Castillo-Tejas J, Castrejón-González O, Alvarado JFJ, Manero O (2014) Prediction of excess pressure drop in contraction-expansion flow by molecular dynamics: Axisymmetric and planar configurations. J Nonnewton Fluid Mech 210:1–11
Materials Design, Inc. Medea
Stukowski A (2009) Visualization and analysis of atomistic simulation data with OVITO-the open visualization tool. Model Simul Mat Sci Eng 18(1):015012
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117(1):1–19
Sun H (1998) Compass: An ab initio force-field optimized for condensed-phase applicationsoverview with details on alkane and benzene compounds. J Phys Chem B 102(38):7338–7364
Price CL, Brooks DJ (2004) A modified tip3p water potential for simulation with ewald summation. J Chem Phys, 121
Ryckaert J-P, Ciccotti G, Berendsen HJC (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23(3):327–341
Nosé Shuichi (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81(1):511–519
Hoover WG (1985) Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A 31:1695–1697
Montes-Zavala I, Castrejón-González EO, Rico-Ramírez V, Pérez E, Santamaría-Razo DA, González-Calderón JA (2020) Which is better? experimental and simulation analyses of the chemical modification of carbon nanotubes to improve their dispersion in water. J Dispers Sci Technol 42(9):1338–1349
Donald A (1965) McQuarry. Statistical Mechanics, Amer Inst Physics
Chen C, Wei S, Xiang B, Wang B, Wang Y, Liang Y, Yuan Y (2019) Synthesis of silane functionalized graphene oxide and its application in anti-corrosion waterborne polyurethane composite coatings. Coatings 9(9):587
Goharshadi EK, Akhlamadi G, Mahdizadeh SJ (2015) Investigation of graphene oxide nanosheets dispersion in water based on solubility parameters: a molecular dynamics simulation study. RSC Adv 5(129):106421–106430
Luo Y, Wang R, Zhao S, Chen Y, Su H, Zhang L, Chan TW, Wu S (2016) Experimental study and molecular dynamics simulation of dynamic properties and interfacial bonding characteristics of graphene/solution-polymerized styrene-butadiene rubber composites. RSC Adv 6(63):58077–58087
Acknowledgements
The authors want to thank the Mexican National Council for Science and Technology (CONACyT) for the scholarship granted to Isidro Montes Zavala. The financial support of TECNM (No. 14887.22-P), and CONACYT (No. INFR-2015-01-254675) is also gratefully acknowledged.
Funding
This work was supported by Mexican National Institute of Technology (Grant no. 14887.22-P), Mexican National Council for Science and Technology (Grant No. INFR-2015-01-254675).
Author information
Authors and Affiliations
Contributions
I. Montes-Zavala developed the simulations and wrote a part of the manuscript. E.O. Castrejón-González wrote the manuscript, discussed the results and was the director of the project. J.A. González-Calderón, reviewed the entire paper and contributed in the results discussions. V. Rico-Ramírez, reviewed the entire paper, contributed in the results discussions and in the general redaction of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montes-Zavala, I., Castrejón-González, E.O., González-Calderón, J.A. et al. Colloidal stability of graphene in aqueous medium: a theoretical approach through molecular dynamics. J Mol Model 29, 220 (2023). https://doi.org/10.1007/s00894-023-05613-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05613-5