Abstract
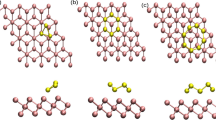
Density functional theory with the addition of a semi-empirical dispersion potential was applied to the conventional Kohn–Sham energy to study the adsorption of alkene and alkyne molecules on hydrogen-terminated two-dimensional group IV systems (graphane, silicane, and germanane) by means of a radical-initiated reaction. In particular, we investigated the interactions of acetylene, ethylene, and styrene with those surfaces. Although we had studied these systems previously, we included van der Waals interactions in all of the cases examined in the present work. These forces, which are noncovalent interactions, can heavily influence different processes in molecular chemistry, such as the adsorption of organic molecules on semiconductor surfaces. This unified approach allowed us to perform a comparative study of the relative reactivities of the various organic molecule/surface systems. The results showed that the degree of covalency of the surface, the lattice size, and the partial charge distribution (caused by differences in electronegativity) are all key elements that determine the reactivity between the molecules and the surfaces tested in this work. The covalent nature of graphane gives rise to energetically favorable intermediate states, while the opposite polarities of the charge distributions of silicane and germanane with the organic molecules favor subsequent steps of the radical-initiated reaction. Finally, the lattice size is a factor that has important consequences due to steric effects present in the systems and the possibility of chain reaction continuation. The results obtained in this work show that careful selection of the substrate is very important. Calculated energy barriers, heats of adsorption, and optimized atomic structures show that the silicane system offers the best reactivity in organic functionalization.








Similar content being viewed by others
References
Hobza P, Müller-Dethlefs (2010) Non-covalent interactions: theory and experiment. Royal Society of Chemistry, Cambridge
Claridge AS, Liao WS, Thomas JC, Zhao Y, Cao HH, Cheunkar S, Serino AC, Andrews AM, Weiss PS (2013) From the bottom up: dimensional control and characterization in molecular monolayers. Chem Soc Rev 42:2725–2745. doi:10.1039/C2CS35365B
Bent SF (2002) Organic functionalization of group IV semiconductor surfaces: principles, examples, applications, and prospects. Surf Sci 500:879–903. doi:10.1016/S0039-6028(01)01553-9
Teplyakov AV, Bent SF (2013) Semiconductor surface functionalization for advances in electronics, energy conversion, and dynamic systems. J Vac Sci Technol A 31:050810-1–050810-12. doi:10.1116/1.4810784
Kamra T, Chaudhary S, Xu C, Montelius L, Schnadt J, Ye L (2016) Covalent immobilization of molecularly imprinted polymer nanoparticles on a gold surface using carbodiimide coupling for chemical sensing. J Colloid Interface Sci 461:1–8. doi:10.1016/j.jcis.2015.09.009
James CD, Davis R, Meyer M, Turner A, Turner S, Withers G, Kam L, Banker G, Craighead H, Isaacson M, Turner J, Shain W (2000) Aligned microcontact printing of micrometer-scale poly-L-lysine structures for controlled growth of cultured neurons on planar microelectrode arrays. IEEE Trans Biomed Eng 47:17–21. doi:10.1109/10.817614
King PH, Corsi JC, Pan BH, Morgan H, de Planque MRR, Zauner KP (2012) Towards molecular computing: co-development of microfluidic devices and chemical reaction media. Biosystems 109:18–23. doi:10.1016/j.biosystems.2012.01.003
De Feyter S, Gesquiere A, Abdel-Mottaleb MM, Grim PCM, De Schryver F, Meiners C, Sieffert M, Valiyaveettil S, Mullen K (2000) Scanning tunneling microscopy: a unique tool in the study of chirality, dynamics, and reactivity in physisorbed organic monolayers. Acc Chem Res 33:520–531. doi:10.1021/ar970040g
De Feyter S, De Schryver F (2003) Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem Soc Rev 32:139–150. doi:10.1039/B206566P
Parsegian VA (2005) Van der Waals forces: a handbook for biologists, chemists, engineers, and physicists. Cambridge University Press, New York
Linford MR, Chidsey CED (1993) Alkyl monolayers covalently bonded to silicon surfaces. J Am Chem Soc 115:12631–12632. doi:10.1021/ja00079a071
Lopinski GP, Wayner DDM, Wolkow RA (2000) Self-directed growth of molecular nanostructures on silicon. Nature 406:48–51. doi:10.1038/35017519
Kang JK, Musgrave CB (2002) A quantum chemical study of the self-directed growth mechanism of styrene and propylene molecular nanowires on the silicon (100) 2×1 surface. J Chem Phys 116:9907. doi:10.1063/1.1476005
Hosssain MZ, Kato HS, Kawai M (2005) Controlled fabrication of 1D molecular lines across the dimer rows on the Si(100)−(2 × 1)−H surface through the radical chain reaction. J Am Chem Soc 127:15030–15031. doi:10.1021/ja055515a
Hosssain MZ, Kato HS, Kawai M (2005) Fabrication of interconnected 1D molecular lines along and across the dimer rows on the Si(100)−(2 × 1)−H surface through the radical chain reaction. J Phys Chem B 109:23129–23133. doi:10.1021/jp055760g
Lee JH, Choi JH, Cho JH (2011) Enhanced stability and electronic structure of phenylacetylene lines on the Si(100)-(2 × 1):H surface. J Phys Chem C 115:14942–14946. doi:10.1021/jp203980y
Hosssain MZ, Kato HS, Jung J, Kim Y, Kawai M (2013) Molecular assembly through the chain reaction of substituted acenes on the Si(100)–(2 × 1)–H surface. J Phys Chem C 117:19436–19441. doi:10.1021/jp405487v
Takeuchi N, Kanai Y, Selloni A (2004) Surface reaction of alkynes and alkenes with H-Si(111): a density functional theory study. J Am Chem Soc 126:15890–15896. doi:10.1021/ja046702w
Takeuchi N, Selloni A (2005) Density functional theory study of one-dimensional growth of styrene on the hydrogen-terminated Si(001)-(3 × 1) surface. J Phys Chem B 109:11967–11972. doi:10.1021/jp0507344
Kanai Y, Takeuchi N, Car R, Selloni A (2005) Role of molecular conjugation in the surface radical reaction of aldehydes with H-Si (111): first principles study. J Phys Chem B 109:18889–18894. doi:10.1021/jp0527610
Takeuchi N, Kanai Y, Selloni A (2010) Surface radical chain reaction revisited: comparative investigation of styrene and 2,4-dimethyl-styrene on hydrogenated Si(001) surface from density functional theory calculations. J Phys Chem C 114:3981–3986. doi:10.1021/jp9097183
Rubio-Pereda P, Takeuchi N (2013) Density functional theory study of the organic functionalization of hydrogenated silicene. J Chem Phys 138:194702. doi:10.1063/1.4804545
Rubio-Pereda P, Takeuchi N (2013) Density functional theory study of the organic functionalization of hydrogenated graphene. J Phys Chem C 177:18738–18745. doi:10.1021/jp406192c
Rubio-Pereda P, Takeuchi N (2015) Adsorption of organic molecules on the hydrogenated germanene: a DFT study. J Phys Chem C 119:27995–28004. doi:10.1021/acs.jpcc.5b08370
Avouris P (2010) Graphene: electronic and photonic properties and devices. Nano Lett 10:4285–4294. doi:10.1021/nl102824h
Dimoulas A (2015) Silicene and germanene: silicon and germanium in the “flatland”. Microelectron Eng 131:68–78. doi:10.1016/j.mee.2014.08.013
Jiang S, Arguilla MQ, Cultrara ND, Goldberger JE (2015) Covalently-controlled properties by design in group IV graphane analogues. Acc Chem Res 48:144–151. doi:10.1021/ar500296e
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. doi:10.1002/jcc.20495
Zhang IY, Xu X (2013) A new generation density functional: towards chemical accuracy for chemistry of main group elements. Springer, Heidelberg
Dzade NY, Roldan A, Leeuw NH (2014) The surface chemistry of NOx on mackinawite (FeS) surfaces: a DFT-D2 study. Phys Chem Chem Phys 16:15444–15456. doi:10.1039/C4CP01138D
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I et al (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21:395502-1–395502-19. doi:10.1088/0953-8984/21/39/395502
Laasonen K, Pasquarello A, Car R, Lee C, Vanderbilt D (1993) Car–Parrinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials. Phys Rev B 47:10142–10153
Perdew JP, Burke K, Ernzerholf M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. doi:10.1103/PhysRevLett.77.3865
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Jensen F (2003) Introduction to computational chemistry. Wiley, Chichester
Emeléus HJ, Stewart K (1935) The oxidation of the silicon hydrides. J Chem Soc 1182–1189. doi: 10.1039/JR9350001182
Acknowledgments
We are grateful for financial support from Conacyt Project 164485 and DGAPA project IN100516. Calculations were performed in the DGCTIC-UNAM supercomputing center (project SC16-1-IG-31).
All of the authors contributed to the writing of the manuscript. All of the authors also gave their approval to the final version of the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1595 kb)
Rights and permissions
About this article
Cite this article
Rubio-Pereda, P., Takeuchi, N. Van der Waals molecular interactions in the organic functionalization of graphane, silicane, and germanane with alkene and alkyne molecules: a DFT-D2 study. J Mol Model 22, 175 (2016). https://doi.org/10.1007/s00894-016-3048-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3048-3