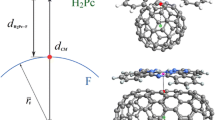
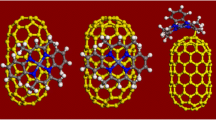
Abstract
Context
Molecular modeling of carbon nanotubes and lanthanide double-decker phthalocyanines hybrids is challenging due to the presence of 4f-electrons. In this paper, we analyzed the trends in structural changes and electronic properties when a lanthanide (La, Gd, and Lu) bisphthalocyanine molecule is adsorbed on the surface of two single-walled carbon nanotubes (SWCNTs) models: armchair and zigzag. The density functional theory (DFT) computations showed that the height of bisphthalocyanines complexes (LnPc2) when adsorbed on a nanotube (LnPc2+SWCNT) is the structural feature which is most affected by the nanotube model. The formation energy of the LnPc2+SWCNT hybrid depends on the metal atom and the nanotube chirality. LaPc2 and LuPc2 bind stronger to the zigzag nanotube, while for GdPc2, bonding to the armchair nanotube is the stronger one. The HOMO-LUMO gap energy (Egap) shows a correlation between the nature of lanthanide and the nanotube chirality. In the case of adsorption on armchair nanotube, Egap tends to match the gap of isolated LnPc2, whereas for adsorption on the zigzag nanotube, it is closer to the value for the isolated nanotube model. The spin density is localized on the phthalocyanines ligands (plus on Gd in the case of GdPc2), when the bisphthalocyanine is adsorbed on the surface of the armchair nanotube. For bonding to zigzag nanotube (ZNT), it extends over both components, except for LaPc2+ZNT, where spin density is found on the nanotube only.
Method
All DFT calculations were carried out using the DMol3 module of Material Studio 8.0 software package from Accelrys Inc. The computational technique chosen was the general gradient approximation functional PBE in combination with a long-range dispersion correction developed by Grimme (PBE-D2), the double numerical basis set DN, and the DFT semi-core pseudopotentials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lanthanide double-decker phthalocyanine complexes (also known as bisphthalocyanines) are composed of (usually) a trivalent lanthanide atom (Ln3+), which is coordinated with a dianionic macrocycle Pc2- and a monoanionic radical ligand Pc•- (that is, [Ln3+(Pc2-)(Pc•-)]; hereafter LnPc2) [1,2,3,4,5]. This class of complexes has attracted great interest due to their remarkable electronic and optical properties, and especially because of their single-molecule magnet (SMM) behavior. In fact, they show a large magnetic anisotropy, slow relaxation of the magnetic moment, and quantum tunneling of magnetization, which makes them promising candidates for applications in spintronics and quantum computing. These molecular quantum magnets offer the spin degree of freedom that can be used to control the charge transport in conducting systems [6, 7].
The self-assembly of LnPc2 on different surfaces is of particular interest, since their deposition compared to transition metal SMMs suggests the survival of a large spin magnetic moment of the rare-earth metal center [8]. LnPc2 complexes have been deposited onto the surfaces of copper (111) [8], gold (111) [9,10,11,12], nickel [13, 14], glass [15], and carbon nanomaterials such as graphene [7, 16], highly oriented pyrolytic graphite (HOPG) [17], and carbon nanotubes (CNTs) [4, 6, 18,19,20,21]. Unfortunately, the properties of SMM films usually change depending on the noble metal and ferromagnetic substrates or the fabrication conditions. The substrate temperature and deposition rate affect the thermodynamics and kinetics for the growth of organic films [15].
In view of their inclusion in spintronic devices, hybrids of LnPc2 with carbon nanomaterials such as graphene and CNTs received special attention, because there the weak spin-orbit coupling is expected to result in long spin coherence lifetimes and lengths [7]. In the case of CNTs, the noncovalent interaction with lanthanide double-decker phthalocyanines (LnPc2+CNTs) thorough π-π stacking is a way to improve the magnetic measurements and bistability of SMMs because the main magnetic properties of rare-earth metal center are preserved [18]. Despite this, LnPc2+CNTs hybrids either obtained by covalent or non-covalent functionalization of the carbon nanotubes surface have been the least explored both experimentally and computationally, contrary to transition metal phthalocyanines (monophthalocyanines, also called single-decker phthalocyanine, MPc; M(II) = Mn, Fe, Co, Ni, Cu, Zn) [4, 6, 18,19,20,21].
In our earlier work [22] dealing with rare-earth double-decker phthalocyanines, we studied the non-covalent interaction of unsubstituted yttrium bisphthalocyanines (YPc2) with single-walled carbon nanotubes (SWCNTs) by density functional theory (DFT; namely, by using the Perdew-Burke-Ernzerhof functional, PBE, and Grimme’s dispersion correction), analyzed structural changes in the two Pc ligands of YPc2 resulting from π-π interaction with the nanotube sidewalls. Other aspects we addressed were the changes in electronic characteristics upon the YPc2 adsorption, the effect of nanotube chirality and of the size of double numerical basis sets available in DMol3 module (DN, DND, and DNP). Compared to MPc and YPc2 hybrids with nanotubes [22,23,24], graphene [25, 26], and fullerenes [27, 28], the same computational task for systems including lanthanide derivatives is computationally much more demanding. As in the case of YPc2, a second large-size C32H16N8 ligand is present, which, when combined with the computational challenge of the 4f electrons from Ce to Lu, and thus with the appearance of a series of highly degenerate states, dramatically complicates the self-consistence field (SCF) convergence.
In order to proceed with DFT studies of non-covalent hybrids of LnPc2 complexes with carbon nanomaterials, it is crucial to obtain the optimized bisphthalocyanine structures, which represent as closely as possible to the ones obtained experimentally by X-ray diffraction (XDR). We focused on this aspect in our previous report [29]. As opposed to what could be logically expected, the larger DND and DNP basis sets (having polarization functions) were found not to be the best choice for the above purpose, due to (often) unresolvable SCF problems and distorted LnPc2 geometries (for example, an eclipsed conformation instead of the typical staggered one). Only the use of a smaller DN basis set helped to complete computations for all lanthanides from La to Lu, as well as to obtain reasonable LnPc2 geometries. Another recent study [30] on other lanthanide-containing systems (endohedral Ln@C60 fullerenes) showed that the use of DN and DND bases yields essentially the same geometrical and electronic features, as with the ytrium bisphthalocyanine system mentioned above. Therefore, to achieve the main goal of the present work, consisting in the analysis of structural changes and electronic properties of LnPc2 phthalocyanines (represented by LaPc2, GdPc2 and LuPc2) in LnPc2+SWCNTs non-covalent hybrids, we employed the DN basis set along with the PBE-D2 functional.
Computational methods
The geometry optimizations and calculations of energies and electronic characteristics of LnPc2+SWCNTs hybrids were performed by using the numerical-based DFT module DMol3 available as part of the Materials Studio 8.0 software from Accelrys, Inc. [31,32,33,34]. The general gradient approximation (GGA) functional by Perdew-Burke-Ernzerhof (PBE) [35] in combination with a long-range dispersion correction by Grimme [36] (PBE-D2) was the computational technique of choice, because dispersion interactions need to be taken into account, when noncovalently bonded molecular systems are analyzed such as the complexes of tetraazamacrocyclic (including porphyrins and Pcs) and many other compounds with fullerene [27, 37], graphene [25, 26] and carbon nanotube models [22,23,24, 38, 39]. Moreover, there are already theoretical studies involving specifically MPc2 complexes that employed this functional [9, 40,41,42,43]. As in a recent study by our group on the optimization of geometry of lanthanide bisphthalocyanines [29], in all calculations, we employed the DFT semi-core pseudopotentials (DSPP; specially designed to use within DMol3 module), which implement relativistic effects and spin-orbit coupling, and the double numerical basis set DN, without polarization functions included (equivalent of 6-31G). A global orbital cutoff was set to 4.3 Å (defined by the presence of Ln atoms), and the convergence criteria were as follows: energy gradient, 10-5 Ha; maximum force, 0.02 Ha/Å; maximum displacement 0.05 Å; SCF tolerance, 10-4; and maximum step size 0.1 Å. As an auxiliary tool to facilitate SCF convergence [29], thermal smearing was used with a target value of 10-4 Ha (equivalent temperature of 31.6 K).
The formation energies ΔELnPc2+SWCNT (hereafter ΔE for simplicity) for the noncovalent hybrids of LnPc2 with SWCNTs models were calculated according to the general equation:
ΔELnPc2+SWCNT = ELnPc2+SWCNT – (ELnPc2 + ESWCNT)
where Ei is the corresponding absolute energy.
Results and discussion
Structural characteristics
To analyze the structural characteristics and electronic properties of LnPc2+SWCNTs hybrids, the geometry of each isolated component was optimized first. Two single-walled carbon nanotube models of different chirality were employed: armchair and zigzag, referred to as ANT and ZNT, which are composed of 180 carbon atoms with 8.23 and 7.67 Å diameter and 17.05 and 18.60 Å length, respectively, and whose ends are capped with fullerene hemispheres (Fig. 1). As representative LnPc2 complexes, we considered the species with a totally empty (LaPc2, electronic configuration [Xe]4f 0), a half-filled (GdPc2, [Xe]4f 7) and a totally filled (LuPc2, [Xe]4f 14) 4f shell. It was impossible to complete the geometry optimization of LnPc2+SWCNTs hybrids without the use of thermal smearing, but the value of 10-4 Ha applied here is very low (equivalent temperature of 31.6 K). At the same time, the structure optimization for LaPc2, GdPc2 and LuPc2 was afforded also with Fermi occupancy [29].
Structural comparison of isolated phthalocyanines and those adsorbed on the surface of nanotubes [22,23,24] and other carbon nanomaterials such as the endohedral fullerene Sc3N@C80 [37] and graphene with defects [25, 26] revealed an important typical feature of these macrocycles, namely, a strong bending distortion of Pc ligands upon interaction, increasing in such a way the area of Pc contact with the latter: in particular, this was observed for free-base H2Pc, its 3d transition metal(II) complexes, as well as yttrium double-decker phthalocyanine interacting with SWCNTs models. This bending due to non-parallel π-π interactions between the two extended π systems occurs to a variable degree, depending on the central atom, the diameter and chirality of the carbon nanotubes [22,23,24, 44, 45]. The structural parameters we used to characterize such a distortion for each LnPc2 in the isolated and the adsorbed state in LnPc2+SWCNTs hybrids (Table 1) are the rotation angle between the two Pc ligands (skew angle; φ), the molecular size (width), height, the N-Ln distance, and the N-Y-N angles. In the case of isolated LnPc2 species, each parameter was compared with the one found in experimentally derived structure XRD from [46, 47] (details of such comparisons were described in Ref. [29]). Also, the formation energies, HOMO, LUMO and HOMO-LUMO gap energies (Egap), the charge and spin of central metal atom (Table 2), as well as the spin density distribution, were analyzed.
As most unsubstituted double-decker phthalocyanines, the ones studied in this work are characterized by a staggered structure (Fig. 1), where the mutual rotation angles between Pc ligands approaches 45° [3, 29]. The corresponding values for the optimized LaPc2 and GdPc2 complexes do not show tangible differences compared to the experimental XRD structures (0.38° and 0.09°, respectively), whereas for LuPc2 this angle differs by 4.9°. This discrepancy can be attributed to the fact that the experimental value refers to the crystalline phase while the theoretical approach considers an isolated molecule, as well as the presence of solvent in the crystal lattice (the reported LuPc2 structure included [NBu4]+ cation, creating very particular chemical environment [47]). In the phthalocyanines adsorbed on the surface of SWCNTs models, the skew angles are not affected: their values vary between 44.8° and 44.9° only, depending on LnPc2 complex and nanotube chirality.
The size (or width) of LnPc2 molecules, which is defined as the maximum distance between the two hydrogen atoms at opposite o-phenylene moieties of Pc rings [11, 48], is overestimated in all cases (Table 1), fluctuating around 15.0 Å. Hence, the length of the nanotube models is barely sufficient to accommodate one LnPc2 molecule. As seen in Table 1, the distance between the hydrogen atoms is not the same for all LnPc2 complexes. Upon adsorption on the SWCNTs models, the o-phenylene moieties are attracted to the nanotube sidewall, leading to a more domed geometry of Pc ligand contacting SWCNTs. The bending distortion is more noticeable when bisphthalocyanines are adsorbed on ZNT, since its diameter is smaller than that of ANT.
It is known that the two Pc ligands of neat LnPc2 complexes are not planar, exhibiting a different degree of bending in the isoindole units (Fig. 1 and Ref. [29]), and this distortion is attributed to the repulsive interaction between the two macrocycles, especially between the o-phenylene rings. A quantitative evaluation of this distortion can be made by analyzing the height of each LnPc2 complex, which is measured as the distance between the peripheral hydrogen atoms belonging to the opposing Pc ligands [11, 49]. The height of La, Gd, and Lu bisphthalocyanine as well as their size (or width) vary within the same molecule, so that it is usually presented as an interval within which the above H...H distances are found (Table 1). For XRD structures of LaPc2, GdPc2, and LuPc2, these intervals are respectively 3.936–4.381 (variation within 0.445 Å), 3.652–4.912 (variation within 1.260 Å), and 3.743–5.141 Å (variation within 1.439 Å). For DFT-optimized LnPc2 geometries, the height variation increases for LaPc2 (from 0.445 to 1.611 Å), but decreases for GdPc2 (from 1.260 to 0.700 Å) and LuPc2 (from 1.439 to 0.652 Å). When LnPc2 complexes interact with SWCNTs models, the molecule height increases, especially in LnPc2+ZNT hybrids (except for GdPc2+ZNT, the H...H distances in decreases). The most noticeable change compared to the height calculated for isolated molecules is observed for La and Lu bisphthalocyanines, for which the height variation increases by about 0.45 Å. This suggests that the height is affected by bending distortion of Pc ligand contacting nanotube sidewall, as a result of strong π-π interactions between the two components.
Another set of parameters, which can be employed to evaluate the distortion of LnPc2, is the length of coordination bonds between the lanthanide and nitrogen atoms of isoindole units (Ln-N). The calculated Ln-N bond lengths in isolated LnPc2 molecules are 2.536–2.545 Å for LaPc2, 2.507–2.529 Å for GdPc2, and 2.384–2.400 Å for LuPc2, and hence larger than the XRD experimental values (Table 1 and Fig. 2). Figure 2 shows how the Ln-N length in each isolated bisphthalocyanine decreases as the Ln atomic number increases, and that this trend is maintained after adsorption on the nanotube sidewall. The N-Ln-N angles in isolated complexes were analyzed as well (Table 1 and Fig. 2), and result underestimated for GdPc2, and overestimated for LaPc2 and LuPc2, compared to the experimental values. The angles of most of the bisphthalocyanines increase after deposition on the surface of each model nanotube with respect to the isolated and optimised structure and reflect greater variation in the range, indicating asymmetry and distortion, the exception of the lanthanum double-decker phthalocyanine on the surface of armchair nanotube, the value of the angles decreases, see Table 1 and Fig. 2. For LnPc2+SWCNTs, the change in N-Ln-N angle is opposite to that of Ln-N bond lengths: the angles increase from La to Lu. The change become more dramatic in the gadolinium hybrids.
Comparison of the Ln-N bond lengths (Å; top) and N-Ln-N angles (°; bottom) in crystalline lanthanide double-decker phthalocyanine complexes obtained by XRD (aLnPc2), in the isolated LnPc2 molecules and adsorbed on the carbon nanotube sidewalls (LnPc2+SWCNTs) calculated at the PBE-D2/DN level of theory
The attraction between the SWCNTs models and LnPc2 complexes can be characterized in terms of the shortest Ln…CSWCNT, γ-N…CSWCNT and CLnPc2…CSWCNT distances. For LaPc2+ANT and GdPc2+ANT closest distance is found between a carbon atom of the nanotube and one of the azomethine nitrogen atoms (γ-N) of the Pc ligand (γ-N...CSWCNT; 3.107 and 3.180 Å, respectively), meanwhile for LuPc2+ANT and all three LnPc2+ZNT hybrids, the closest contact is between carbon atoms, CLnPc2…CSWCNT. The shortest distance between lanthanide and a carbon atom of the nanotube (Ln...CSWCNT; Table 1) is one of the structural parameters that is most sensible to the nanotube model and the Ln species. In the LnPc2+ANT series, this distance increases as the lanthanide atomic number increases, from 4.551 Å for LaPc2+ANT to 4.619 Å for LuPc2+ANT, while an opposite behavior is observed for the LnPc2+ZNT series, where it decreases from 4.692 Å for LaPc2+ZNT to 4.533 Å for LuPc2+ZNT.
It is important to mention that the use of electron smearing technique, as a tool to solve the SCF convergence problems [29, 50,51,52], does not affect the geometry features for isolated LnPc2 complexes, compared to those computed using Fermi occupancy, when the smearing values are as low as (1-5)x10-4 Ha.
Adsorption strength and electronic properties
From Table 2, one can see that the complex formation energy (or adsorption energy) depends on the nature of metal. The lowest negative ΔE values of −65.6 and −64.6 kcal/mol were obtained for GdPc2+ANT and GdPc2+ZNT, respectively, indicative of the strongest binding. For both the ANT and the ZNT series, ΔE increases in the order of GdPc2 < LuPc2 < LaPc2. Concerning the effect of the nanotube chirality, LaPc2 and LuPc2 adsorbed on ZNT show more negative energies than the ones adsorbed on ANT: −55.4 and −60.7 kcal/mol vs. −52.4 and −55.1 kcal/mol, respectively. At the same, an opposite trend can be seen for GdPc2+SWCNTs hybrids, though the difference is as small as 1 kcal/mol. In this regard, it is interesting to mention that LaPc2+SWCNTs behave similarly to their YPc2 analogues [22], where the central rare-earth metal has no f-orbitals, and the nanotube models were substantially smaller.
We also calculated HOMO, LUMO and HOMO-LUMO gap energies (Table 2), and analyzed the corresponding frontier orbital plots (Fig. 3). The gap energy for isolated LnPc2 complexes slightly decreases in the order of LuPc2 (0.138 eV)> LaPc2 (0.133 eV)> GdPc2 (0. 130 eV), as in earlier calculations with Fermi occupancy [22]. Among the nanotube models, ANT exhibits a higher band gap than ZNT (0.551 and 0.001 eV, respectively), similarly to the smaller nanotube models with the same chirality used to study their non-covalent interactions with 3d transition metal(II) MPcs [22, 23, 39] and YPc2 [24]. For what concerns the gap energy of the LnPc2+SWCNTs hybrids, the following observations can be made. Firstly, for LnPc2+ANT hybrids Egap changes linearly with the lanthanide atomic number. The gap becomes slightly larger as the atomic number, and consequently the number of 4f-electrons increases: 0.128 eV for LaPc2, 0.131 eV for GdPc2, and 0.134 eV for, LuPc2. For LnPc2+ZNT, trend is opposite, but the Egap values are smaller by one order of magnitude: 0.021, 0.014 and 0.012 eV for LaPc2, GdPc2 and LuPc2, respectively. Secondly, comparing the computed gap values of each hybrid with that of the isolated component (Table 2), one can conclude that in the case of LnPc2+ANT, Egap tends to approach the one of the respective isolated LnPc2, whereas in the LnPc2+ZNT series, it is closer to the band gap of the nanotube, a feature that was found also for YPc2+SWCNTs dyads [24]. The fact that the gap energy is higher for LnPc2+ANT than for LnPc2+ZNT dyads, also observed in our earlier studies of hybrids with 3d transition metal(II) MPcs [22, 23, 39] and YPc2 [24], can be interpreted as an effect of the nanotube chirality. At the same time, our theoretical band gap values should be taken with a certain precaution, since it is known that they are strongly underestimated when using pure GGA functionals (PBE in particular).
As far as the distribution of frontier orbitals is concerned, Fig. 3 and Fig. S1 illustrate that for isolated LaPc2, GdPc2, and LuPc2, the HOMO and LUMO is localized on the carbon atoms of the macrocycle, specifically at the pyrrole unit, as observed earlier for YPc2 [24] and LnPc2 [29] by us and by other research groups at different theoretical levels [40, 53]. For LnPc2+SWCNTs hybrids, its behavior depends on the nanotube chirality and on the central Ln atom (Fig. 3 and Fig. S2). In the hybrids with ANT, HOMO and LUMO are localized on bisphthalocyanine as in isolated LnPc2 and in YPc2+ANT [22]. In LnPc2+ZNT, the frontier orbital distribution varies. In LaPc2+ZNT and LuPc2+ZNT, HOMO is located exclusively on nanotube, and LUMO on both components; in the case of LuPc2+ZNT, the contribution from the nanotube is more notable. In GdPc2+ZNT, HOMO extends over both components and LUMO is localized only nanotube, similarly to the case of YPc2+ZNT [22]. An additional detail, which can be observed in Fig. 3, is that neither HOMO nor LUMO is localized on the central Ln metal.
One more aspect of interest we addressed is the charge of lanthanide atom (Table 2), as estimated from the Mulliken population analysis. The charge of La, Gd, and Lu in isolated bisphthalocyanines is 1.827, 1.452, and 1.400 e, respectively. In the case of hybridss, the changes are rather random. For LaPc2+SWCNTs hybrids, there is an increase by 0.031 e for LaPc2+ANT and 0.099 e for LaPc2+ZNT. For their GdPc2+ANT and GdPc2+ZNT, the Gd charge decreases by 0.045 and 0.060 e, respectively. For LuPc2+SWCNTs hybrids, the Lu charge increases by 0.019 e for LuPc2+ANT but decreases insignificantly, by 0.007 e for LuPc2+ZNT. Regardless of the magnitude, the general trend the same as for isolated phthalocyanines, where the Ln charge decreases in the order of LaPc2 > GdPc2 > LuPc2.
The trend of charge transfer within the hybrids was analyzed since carbon nanotubes and phthalocyanine hybrids have been considered as supramolecular self-assembled donor-acceptor conjugated systems. From Table 2, it is clear that the direction of charge transfer is from the phthalocyanine to the carbon nanotube and is influenced by the chirality of the nanotube and the central coordination metal. For phthalocyanines adsorbed on the surface of armchair nanotubes, the charge transfer increases inversely to the lanthanide atomic number, from 0.079 (LuPc2+ANT) to 0.090 e (LaPc2+ANT), while for zigzag nanotube hybrids, it increases directly from 0.377 (LaPc2+ZNT) to 0.502 e (LuPc2+ZNT), and the latter hybrids apparently generate a higher charge transfer. Something particular that can be denoted and associated, is the Ln…CSWCNT distance, for each set of hybrids per chirality, which has opposite behavior to that structural parameter (Table 1), and that is that the smaller the Ln…CSWCNT distance, the higher the charge transfer.
Spin density plots calculated for the isolated LnPc2 complexes, the SWCNTs models, and the LnPc2+SWCNTs hybrids are presented in Fig. 4 (also in Figs. S1 and S2). The distribution of the spin density in isolated LaPc2 and LuPc2 matches closely the HOMO and LUMO distribution discussed above (Fig. 3). In these complexes, the unpaired electrons are found mainly on carbon atoms of the pyrrole unit that are bonded with the nitrogen atoms, as well as a minor contribution from γ-N and isoindole N atoms. This feature is also present in GdPc2, but the additional main contribution here comes from the metal.
Spin density plots for lanthanides double-decker phthalocyanine (LaPc2, GdPc2 and LuPc2;), SWCNTs models, and LnPc2+SWCNTs hybrids (isosurfaces at 0.01 a.u;) calculated by using the PBE GGA functional with Grimme’s dispersion correction with the DN basis set. Violet and orange lobes correspond to spin-up and spin-down electrons, respectively
The spin distribution in the hybrids depends not only on the central metal, but also on the nanotube model. The plots for the LnPc2+ANT hybrids very similar to those of the isolated LnPc2 complexes, while those for the three hybrids with ZNT exhibit notable differences. In all of them, one can observe the presence of unpaired electrons on the closed nanotube ends (as in the isolated ZNT model). No tangible contribution from the bisphthalocyanine can be found in LaPc2+ZNT, and only a minor one in that of LuPc2+ZNT. This is in contrast to GdPc2+ZNT, where the spin density distribution of the isolated GdPc2 and of ZNT is combined, in the latter the contribution of spin up (violet lobule) and spin down (orange lobule) in the complex is reversed when deposited in armchair nanotubes. Although qualitatively no differences are observed in the spin density of the ligands of each phthalocyanine on the nanotubes, quantitatively it can be deduced that for LaPc2 on both nanotubes, the ligand that is closer to the nanomaterial wall has a lower density (for ANT 0.441 vs. 0.575 e and for ZNT-0.003 vs. 0.022 e) opposite to the behavior of GdPc2 (for ANT -0.445 vs. 0.569 e and for ZNT -0.217 vs. -0.239 e). Meanwhile, LuPc2 adsorbed on ANT the ligand has a lower density 0.419 vs. 0.533 e) and on ZNT a higher density (-0.113 vs. -0.143 e).
Table 2 specifies also the spin of Ln atoms in isolated and adsorbed double-decker phthalocyanines. One can see that the Ln spin remains relatively constant. For LaPc2 and LuPc2 complexes, where the lanthanide(III) ion is in a closed-shell configuration, it is always close to zero. On the other hand, for GdPc2 where the 4f orbital of gadolinium ion is half-filled, a minor spin transfer of 0.004 and 0.007 e from ANT and ZNT, respectively, was found.
Conclusions
The main results can be summarized as follows:
-
The height of LnPc2 complexes adsorbed on nanotubes is one of the structural features which is most affected by the SWCNTs diameter; the distance between H atoms of opposite Pc ligands increases stronger more for the nanotube with the smaller-diameter, because the ligands are more strongly bent and take on a domed geometry.
-
Ln-N bond length and N-Ln-N angle of lanthanides bisphthalocyanines on nanotubes with both armchair and zigzag follow the same trend as in isolated LnPc2, the Ln-N bond length decreases with increasing atomic number of the central metal, while the value of N-Ln-N increases.
-
The formation energy of LnPc2+SWCNTs hybrids depends on the type of lanthanide and the nanotube chirality. LaPc2 and LuPc2 bond stronger to the nanotube with armchair chirality than to the zigzag tube, while the opposite occurs in the case of GdPc2 (though the difference is insignificant).
-
The HOMO-LUMO gap width correlates with the number of electrons of lanthanide and nanotube chirality. For LnPc2+ANT hybrids Egap increases linearly in the order LaPc2 < GdPc2 < LuPc2, whereas LnPc2+ZNT hybrids the trend is opposite and Egap decreases in the order LaPc2 > GdPc2 > LuPc2. Hybrids resulting from the adsorption on the armchair nanotube have a larger gap compared to the case when LnPc2 binds to zigzag tube. In the former case, Egap tends to match the gap of isolated LnPc2, whereas in the latter case it is closer to the value for zigzag tube alone.
-
The spin density for LnPc2 adsorbed on the armchair nanotube is localized on the Pc ligands, for LnPc2 adsorbed on the zigzag nanotube on both interaction components, and for LaPc2 deposited on the zigzag tube only on nanotube.
References
Vitali L, Fabris S, Mosca Conte A et al Electronic structure of surface-supported Bis(phthalocyaninato) terbium(III). Single Mol Magn. https://doi.org/10.1021/nl801869b
Serrano G, Wiespointner-Baumgarthuber S, Tebi S et al (2016) Bilayer of terbium double-decker single-molecule magnets. J Phys Chem C 120:13581–13586. https://doi.org/10.1021/acs.jpcc.6b03676
Woodruff DN, Winpenny REP, Layfield RA (2013) Lanthanide single-mol mag. Chem Rev 113:5110–5148. https://doi.org/10.1021/CR400018Q
Apetrei C, Nieto M, Rodríguez-Méndez ML, De Saja JA (2011) Development of lutetium bisphthalocyanine/carbon nanotube Langmuir-Blodgett films. Sensing Properties J Porphyr Phthalocyanines 15:908–917. https://doi.org/10.1142/S108842461100377X
Feltham HLC, Brooker S (2014) Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide ion. Coord Chem Rev 276:1–33. https://doi.org/10.1016/j.ccr.2014.05.011
Urdampilleta M, Klyatskaya S, Cleuziou JP et al (2011) Supramolecular spin valves. Nat Mater 10:502–506. https://doi.org/10.1038/nmat3050
Candini A, Klyatskaya S, Ruben M et al (2011) Graphene spintronic devices with molecular nanomagnets. Nano Lett 11:2634–2639. https://doi.org/10.1021/nl2006142
Stepanow S, Honolka J, Gambardella P et al (2010) Spin and orbital magnetic moment anisotropies of monodispersed bis(Phthalocyaninato)terbium on a copper surface. J Am Chem Soc 132:11900–11901. https://doi.org/10.1021/ja105124r
Komeda T, Katoh K, Yamashita M (2014) Double-decker phthalocyanine complex: Scanning tunneling microscopy study of film formation and spin properties. Prog Surf Sci 89:127–160. https://doi.org/10.1016/j.progsurf.2014.03.001
Zhang YF, Isshiki H, Katoh K et al (2009) Low-Temperature scanning tunneling microscopy investigation of bis(phthalocyaninato)yttrium growth on Au(111): from individual molecules to two-dimensional domains. J Phys Chem C 113:9826–9830. https://doi.org/10.1021/jp902410q
Katoh K, Yoshida Y, Yamashita M et al (2009) Direct observation of lanthanide(III)-phthalocyanine molecules on Au(111) by using scanning tunneling microscopy and scanning tunneling spectroscopy and thin-film field-effect transistor properties of Tb(III)- and Dy(III)-phthalocyanine molecules. J Am Chem Soc 131:9967–9976. https://doi.org/10.1021/ja902349t
Fu Y-S, Rg J, Bel S et al (2012) Reversible chiral switching of bis(phthalocyaninato) Terbium(III) on a Metal Surface. Nano Lett 12:3935. https://doi.org/10.1021/nl302166z
Lodi Rizzini A, Krull C, Balashov T et al (2011) Coupling single molecule magnets to ferromagnetic substrates. Phys Rev Lett 107:1–5. https://doi.org/10.1103/PhysRevLett.107.177205
Klar D, Klyatskaya S, Candini A et al (2013) Antiferromagnetic coupling of TbPc2 molecules to ultrathin Ni and Co films. Beilstein J Nanotechnol 436(4):320–324. https://doi.org/10.3762/BJNANO.4.36
Ruan L, Tong J, Luo F et al (2022) The magnetic anisotropy of Tb-phthalocyanine films effected by molecular orientation. Appl Surf Sci 585:152445. https://doi.org/10.1016/j.apsusc.2022.152445
Corradini V, Candini A, Klar D et al (2018) Probing magnetic coupling between LnPc2 (Ln = Tb, Er) molecules and the graphene/Ni (111) substrate with and without Au-intercalation: Role of the dipolar field. Nanoscale 10:277–283. https://doi.org/10.1039/c7nr06610d
Gonidec M, Biagi R, Corradini V et al (2011) Surface supramolecular organization of a terbium(III) double-decker complex on graphite and its single molecule magnet behavior. J Am Chem Soc 133:6603–6612. https://doi.org/10.1021/ja109296c
Kyatskaya S, Mascarós JRG, Bogani L et al (2009) Anchoring of rare-earth-based single-molecule magnets on single-walled carbon nanotubes. J Am Chem Soc 131:15143–15151. https://doi.org/10.1021/ja906165e
Abdullah K, Kong X, Imran M et al (2019) Excellent ambipolar gas sensing response of Eu[Pc(OC4 H9)8]2/acidified multiwalled carbon nanotubes hybrid at room temperature. J Porphyrins Phthalocyanines 23:1455–1462. https://doi.org/10.1142/S1088424619501554
Xu HB, Chen HZ, Shi MM et al (2005) A novel donor–acceptor heterojunction from single-walled carbon nanotubes functionalized by erbium bisphthalocyanine. Mater Chem Phys 94:342–346. https://doi.org/10.1016/J.MATCHEMPHYS.2005.04.056
Katoh K, Sato J, Nakanishi R et al (2021) Terbium(III) bis-phthalocyaninato single-molecule magnet encapsulated in a single-walled carbon nanotube. 9. https://doi.org/10.1039/d1tc01026c
Basiuk EV, Huerta L, Basiuk VA (2019) Noncovalent bonding of 3d metal(II) phthalocyanines with single-walled carbon nanotubes: a combined DFT and XPS study. Appl Surf Sci 470:622–630. https://doi.org/10.1016/j.apsusc.2018.11.159
Bolivar-Pineda LM, Basiuk V (2020) Interactions of metal phthalocyanines with Stone-Wales defects on single-walled carbon nanotubes : a theoretical study. J Appl Phys 127:025302. https://doi.org/10.1063/1.5128629
Bolivar-Pineda LM, Sinecio-Ontiveros MI, Basiuk VA (2021) Distortion of yttrium bisphthalocyanine (YPc2) upon noncovalent interaction with carbon nanotubes: a DFT study. Mater Today Commun 28:102667. https://doi.org/10.1016/J.MTCOMM.2021.102667
Mendoza-Domínguez CU, Basiuk VA (2021) Distortion and bonding strength of phthalocyanine molecules adsorbed on topological defects in graphene. Mater Chem Phys 271:124963. https://doi.org/10.1016/J.MATCHEMPHYS.2021.124963
Mendoza-Domínguez CU, Basiuk VA (2022) Adsorption of yttrium bisphthalocyanine on pristine and defect-contaning graphene models: A DFT study. https://doi.org/10.1016/j.diamond.2022.109051
Tahuilan-Anguiano DE, Basiuk VA (2021) Complexation of free-base and 3d transition metal(II) phthalocyanines with endohedral fullerenes H@C60, H2@C60 and He@C60: The effect of encapsulated species. Diam Relat Mater 118:108510. https://doi.org/10.1016/J.DIAMOND.2021.108510
Tahuilan-Anguiano DE, Basiuk VA (2022) Interaction of free-base and 3d metal(II) phthalocyanines with open-shell endohedral fullerenes species N@C60 and P@C60. Diam Relat Mater 126:109075. https://doi.org/10.1016/J.DIAMOND.2022.109075
Martínez-Flores C, Bolívar-Pineda LM, Basiuk VA (2022) Lanthanide bisphthalocyanine single-molecule magnets : a DFT survey of their geometries and electronic properties from lanthanum to lutetium. Mater Chem Phys 287:126271. https://doi.org/10.1016/j.matchemphys.2022.126271
Martínez-Flores C, Basiuk VA (2022) Ln@C60 endohedral fullerenes: a DFT analysis for the complete series from lanthanum to lutetium. Comput Theor Chem 1217:113878. https://doi.org/10.1016/j.comptc.2022.113878
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules 92:508–517. https://doi.org/10.1063/1.458452
Delley B (1996) Fast calculation of electrostatics in crystals and large molecules. J Phys Chem 100:6107–6110. https://doi.org/10.1021/JP952713N
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764. https://doi.org/10.1063/1.1316015
Delley B, Ellis DE, Freeman AJ et al (1983) Binding energy and electronic structure of small copper particles. Phys Rev B 27:2132–2144. https://doi.org/10.1103/PhysRevB.27.2132
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Grimme S (2006) Semiempirical GGA - type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc
Basiuk VA, Tahuilan-Anguiano DE (2019) Complexation of free-base and 3d transition metal(II) phthalocyanines with endohedral fullerene Sc3N@C80. Chem Phys Lett 722:146–152. https://doi.org/10.1016/j.cplett.2019.03.019
Basiuk VA, Chávez-colorado E (2019) Adsorption of free-base phthalocyanine on Stone-Wales defect-containing carbon nanotubes : a DFT study. Diam Relat Mater 97:107443. https://doi.org/10.1016/j.diamond.2019.107443
Basiuk VA, Bolivar-Pineda LM, Meza-Laguna V et al (2018) Carbon nanotubes and graphene promote pyrolysis of free-base phthalocyanine. J Phys Chem Lett 9:4420–4427. https://doi.org/10.1021/acs.jpclett.8b02141
Farronato M, Bidermane I, Lüder J et al (2018) New quadratic self-assembly of double-decker phthalocyanine on gold(111) surface: from macroscopic to microscopic scale. J Phys Chem C 122:26480–26488. https://doi.org/10.1021/ACS.JPCC.8B08462/SUPPL_FILE/JP8B08462_SI_001.PDF
Komeda T, Isshiki H, Liu J et al (2011) Observation and electric current control of a local spin in a single-molecule magnet. Nat Commun 2. https://doi.org/10.1038/ncomms1210
Mannini M, Bertani F, Tudisco C, et al (2014) Magnetic behaviour of TbPc2 single-molecule magnets chemically grafted on silicon surface. Nat Commun 2014 51 5:1–8. https://doi.org/10.1038/ncomms5582
Pushkarev VE, Tolbin AY, Zhurkin FE et al (2012) Sandwich double-decker Lanthanide(III) “Intracavity” complexes based on clamshell-type phthalocyanine ligands: synthesis, spectral, electrochemical, and spectroelectrochemical investigations. Chem – A Eur J 18:9046–9055. https://doi.org/10.1002/CHEM.201200361
Tian P, Zhang B, Chen J et al (2021) Curvature-induced electronic tuning of molecular catalysts for CO2 reduction. Cat Sci Technol. https://doi.org/10.1039/d0cy01589j
Basiuk VA, Flores-Sánchez LJ, Meza-Laguna V et al (2018) Noncovalent functionalization of pristine CVD single-walled carbon nanotubes with 3d metal(II) phthalocyanines by adsorption from the gas phase. Appl Surf Sci 436:1123–1133. https://doi.org/10.1016/j.apsusc.2017.12.122
Dailey M, Besson C (2021) Selective crystallization of four bis(phthalocyaninato)lanthanoid(III) polymorphs. CrystEngComm 23:7151–7161. https://doi.org/10.1039/d1ce00936b
Moussavi M, De Cian A, Fischer J, Weiss R (1988) Synthesis, structure, and spectroscopic properties of the reduced and reduced protonated forms of lutetium diphthalocyanine. Inorg Chem 27:1287–1291. https://doi.org/10.1021/ic00280a040
Kahlal S, Mentec A, Pondaven A et al (2009) Substituent effect in unsymmetrical lutetium bisphthalocyanines: A DFT analysis. New J Chem 33:574–582. https://doi.org/10.1039/b810131k
Kratochvílová I, Šebera J, Paruzel B et al (2018) Electronic functionality of Gd-bisphthalocyanine: charge carrier concentration, charge mobility, and influence of local magnetic field. Synth Met 236:68–78. https://doi.org/10.1016/J.SYNTHMET.2018.01.007
Basiuk VA (2011) Electron smearing DFT calculations: a case study ofo doxorubicin interaction with single-walled carbon nanotubes. Int J Quantum Chem 111:4197–4205. https://doi.org/10.1002/qua
Basiuk VA, Prezhdo OV, Basiuk EV (2020) Thermal smearing in DFT calculations: How small is really small? A case of La and Lu atoms adsorbed on graphene. Mater Today Commun 25:101595. https://doi.org/10.1016/J.MTCOMM.2020.101595
Basiuk VA, Acevedo-Guzmán DA, Meza-Laguna V et al (2021) High-energy ball-milling preparation and characterization of Ln2O3−graphite nanocomposites. Mater Today Commun 26:102030. https://doi.org/10.1016/J.MTCOMM.2021.102030
Barhoumi R, Amokrane A, Klyatskaya S et al (2019) Screening the 4f-electron spin of TbPc2 single-molecule magnets on metal substrates by ligand channeling. Nanoscale 11:21167–21179. https://doi.org/10.1039/C9NR05873G
Acknowledgements
Financial support from the National Autonomous University of Mexico (UNAM, grant DGAPA- IN103622) is greatly appreciated. L. M. B.-P. is indebted to the Doctorate Degree Program in Chemical Sciences of UNAM, to CONACyT and to the Double-Degree Program of UNAM with the University of Groningen for PhD scholarship. C.U.M.-D. thanks the Doctorate Degree Program in Chemical Sciences of UNAM and CONACyT for a PhD scholarship. The authors thank Prof. Petra Rudolf (Zernike Institute for Advanced Materials, University of Groningen) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
Lina M. Bolivar-Pineda: Investigation, Data curation, Formal analysis, Validation, Visualization, Writing-original draft.
Carlos Uriel Mendoza-Domínguez: Investigation, Data curation, Formal analysis.
Vladimir A. Basiuk: Conceptualization, Methodology, Supervision, Writing-review & editing, Funding.
Corresponding authors
Ethics declarations
Ethical approval
No applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 6684 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bolívar-Pineda, L.M., Mendoza-Domínguez, C.U. & Basiuk, V.A. Adsorption of lanthanide double-decker phthalocyanines on single-walled carbon nanotubes: structural changes and electronic properties as studied by density functional theory. J Mol Model 29, 158 (2023). https://doi.org/10.1007/s00894-023-05557-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05557-w