Abstract
Context
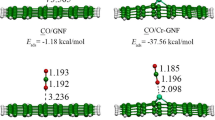
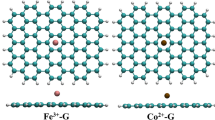
In this research, CO2 and NO2 adsorption on doped nanographene (NG) sheets with transition metals (Fe, Ni, Zn) and (Mn, Co, Cu), respectively, have been applied for scavenging of these toxic gases as the environmental pollutants. The values of changes of atomic charge density have illustrated a more significant charge transfer for Ni-doped C-NG through CO2 adsorption and a more remarkable charge transfer for Co-doped C-NG through NO2 adsorption. The data of NMR spectroscopy has depicted several fluctuations around the graph of Zn-doped on the nanographene surface. The thermodynamic results from IR spectroscopy have indicated that \(\Delta {G}_{\textrm{ads},\textrm{NO}2\to \textrm{TM}@\textrm{C}-\textrm{NG}}^{\textrm{o}}\) values are almost similar for doped metal transitions of Mn, Co, and Cu on the C-NG nanosheet, while \(\Delta {G}_{\textrm{ads},\kern0.5em \textrm{CO}2\to \textrm{TM}@\textrm{C}-\textrm{NG}}^{\textrm{o}}\) has the largest gap of Gibbs free energy adsorption with dipole moment.
Methods
The Langmuir adsorption model with a three-layered ONIOM using CAM-B3LYP functional accompanying LANL2DZ, EPR-III and 6-31 + G (d,p) basis sets due to Gaussian 16 revision C.01 program on the complexes of CO2 → (Fe, Ni, Zn) and NO2 → (Mn, Co, Cu) doped on the C-NG has been accomplished. Then, NMR and IR spectroscopy, nuclear quadrupole resonance, and natural bond orbital analysis have been accomplished for evaluating chemical shielding tensors, thermodynamic properties, electric potential, and occupancy fluctuation through bond orbitals, respectively. In addition, frontier orbitals of LUMO, HOMO, and also a series of chemical reactivity parameters have been calculated. Finally, time-dependent-DFT method due to UV-VIS spectrums has been accomplished to discern the low-lying excited states of CO2 and NO2 adsorption on the (Fe, Ni, Zn) and (Mn, Co, Cu), respectively, doped C-NG sheet.











Similar content being viewed by others
Data availability
It is not applicable.
References
Su Y, Wang J, Wang B, Yang T, Yang B, Xie G, Zhou Y, Zhang S, Tai H, Cai Z et al (2020) Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 14:6067–6075. https://doi.org/10.1021/acsnano.0c01804
Ma D, Zhang J, Li X, He C, Lu Z, Lu Z, Lu Z, Yang Z, Wang Y (2018) C3N monolayers as promising candidates for NO2 sensors. Sens Actuators B Chem 266:664–673. https://doi.org/10.1016/j.snb.2018.03.159
Pacheco M, Pacheco J, Valdivia R, Santana A, Tu X, Mendoza D, Frias H, Medina L, Macias J (2017) Green applications of carbon nanostructures produced by plasma techniques. MRS Adv 2:2647–2659
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163
Nasibulin AG, Pikhitsa PV, Jiang H, Brown DP, Krasheninnikov AV, Anisimov AS, Queipo P, Moisala A, Gonzalez D, Lientschnig G et al (2007) A novel hybrid carbon material. Nat Nanotechnol 2:156–161
Moisala A, Nasibulin AG, Shandakov SD, Jiang H, Kauppinen EI (2005) On-line detection of single-walled carbon nanotube formation during aerosol synthesis methods. Carbon 43:2066–2074
Delgado JL, Herranz M, Martín N (2008) The nano-forms of carbon. J Mater Chem 18:1417
Falcao EH, Wudl F (2007) Carbon allotropes: beyond graphite and diamond. J Chem Technol Biotechnol 82:524–531
Langenhorst F, Campione M (2019) Ideal and real structures of different forms of carbon, with some remarks on their geological significance. J Geol Soc 176:337–347
Louis H, Patrick M, Amodu IO, Benjamin I, Ikot IJ, Iniama GE, Adeyink AS (2023) Sensor behavior of transition-metals (X = Ag, Au, Pd, and Pt) doped Zn11-X-O12 nanostructured materials for the detection of serotonin. Mater Today Commun 34:105048. https://doi.org/10.1016/j.mtcomm.2022.105048
Odey DO, Edet HO, Louis H, Gber TE, Nwagu AD, Adalikwu SA, Adeyinka AS (2023) Heteroatoms (B, N, and P) doped on nickel-doped graphene for phosgene (COCl2) adsorption: insight from theoretical calculations. Mater Today Sustain 21:100294. https://doi.org/10.1016/j.mtsust.2022.100294
Louis H, Charlie DE, Amodu IO, Benjamin I, Gber TE, Agwamba EC, Adeyinka AS (2022) Probing the reactions of thiourea (CH4N2S) with metals (X = Au, Hf, Hg, Ir, Os, W, Pt, and Re) anchored on fullerene surfaces (C59X). ACS Omega 7(39):35118–35135. https://doi.org/10.1021/acsomega.2c04044
Lee SW, Lee W, Hong Y, Lee G, Yoon DS (2018) Recent advances in carbon material-based NO2 gas sensors. Sens Actuators B Chem 255:1788–1804. https://doi.org/10.1016/j.snb.2017.08.203
Chatterjee SG, Chatterjee S, Ray AK, Chakraborty AK (221) Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens Actuators B Chem:1170–1181
Xiao Z, Kong LB, Ruan S, Li X, Yu S, Li X, Jiang Y, Yao Z, Ye S, Wang C et al (2018) Recent development in nanocarbon materials for gas sensor applications. Sens Actuators B Chem 274:235–267. https://doi.org/10.1016/j.snb.2018.07.040
Monajjemi M, Baie MT, Mollaamin F (2010) Interaction between threonine and cadmium cation in [Cd(Thr)] (n = 1–3) complexes: density functional calculations. Russ Chem Bull 59:886–889
Khalili Hadad B, Mollaamin F, Monajjemi M (2011) Biophysical chemistry of macrocycles for drug delivery: A theoretical study. Russ Chem Bull 60:238–241
Monajjemi M, Khaleghian M, Tadayonpour N, Mollaamin F (2010) The effect of different solvents and temperatures on stability of single-walled carbon nanotube: A QM/MD study. Int J Nanosci 09:517–529
Bakhshi K, Mollaamin F, Monajjemi M (2011) Exchange and correlation effect of hydrogen chemisorption on nano V(100) surface: a DFT study by generalized gradient approximation (GGA). J Comput Theor Nanosci 8:763–768. https://doi.org/10.1166/jctn.2011.1750
Mollaamin F, Ilkhani A, Sakhaei N, Bonsakhteh B, Faridchehr A, Tohidi S, Monajjemi M (2015) Thermodynamic and solvent effect on dynamic structures of nano bilayer-cell membrane: hydrogen bonding study. J Comput Theor Nanosci 12:3148–3154. https://doi.org/10.1166/jctn.2015.4092
Adanna D, Nwagu AD, Louis H, Edet HO, Benjamin I, Osabor VN, Adeyinka AS (2023) Computational study on nickel doped encapsulated Mg, K, Ca on pristine C24 nanocage for gas sensing applications. Mater Sci Semicond Process 157:107334. https://doi.org/10.1016/j.mssp.2023.107334
Mohammadi MD, Abbas F, Louis H, Mathias GE, Unimuke TO (2022) Trapping of CO, CO2, H2S, NH3, NO, NO2, and SO2 by polyoxometalate compound. Comput Theor Chem 1215:113826. https://doi.org/10.1016/j.comptc.2022.113826
Louis H, Egemonye TGC, Unimuke TO, Inah BE, Edet HO, Eno EA, Adalikwu SA, Adeyinka AS (2022) Detection of carbon, sulfur, and nitrogen dioxide pollutants with a 2D Ca12O12 nanostructured Material. ACS Omega 7(39):34929–34943. https://doi.org/10.1021/acsomega.2c03512
Louis H, Amodu IO, Unimuke TO, Gber TE, Isang BB, Adeyinka AS (2022) Modeling of Ca12O12, Mg12O12, and Al12N12 nanostructured materials as sensors for phosgene (Cl2CO). Mater Today Commun 32:103946. https://doi.org/10.1016/j.mtcomm.2022.103946
Mollaamin F, Monajjemi M (2022) Molecular modelling framework of metal-organic clusters for conserving surfaces: Langmuir sorption through the TD-DFT/ONIOM approach. Mol Simul 49:365–376. https://doi.org/10.1080/08927022.2022.2159996
Mollaamin F, Shahriari S, Monajjemi M, Khalaj Z (2022) Nanocluster of aluminum lattice via organic inhibitors coating: a study of Freundlich adsorption. J Clust Sci:1–16. https://doi.org/10.1007/s10876-022-02335-1
Hanaor DAH, Ghadiri M, Chrzanowski W, Gan Y (2014) Scalable surface area characterization by electrokinetic analysis of complex anion adsorption (PDF). Langmuir 30(50):15143–15152. https://doi.org/10.1021/la503581e
Boyd A, Dube I, Fedorov G, Paranjape M, Barbara P (2014) Gas sensing mechanism of carbon nanotubes: from single tubes to high-density networks. Carbon 69:417–423
Zhao J, Buldum A, Han J, Lu JP (2002) Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 13:195–200
Svensson M, Humbel S, Froese RDJ, Matsubara T, Sieber S, Morokuma K (1996) ONIOM: A multilayered integrated MO + MM method for geometry optimizations and single point energy predictions. A test for Diels−Alder reactions and Pt(P(t-Bu)3)2 + H2 oxidative addition. J Phys Chem 100(50):19357–19363. https://doi.org/10.1021/jp962071j
Brandt F, Jacob CR (2022) Systematic QM region construction in QM/MM calculations based on uncertainty quantification. J Chem Theory Comput 18(4):2584–2596. https://doi.org/10.1021/acs.jctc.1c01093
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Grimme S, Antony J, Ehrlich S, Krieg H (2010). J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys. 54:724–728
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV et al (2016) Gaussian 16, Revision C.01. Gaussian, Inc., Wallingford CT
Fry RA, Kwon KD, Komarneni S, Kubicki JD, Mueller KT (2006) Solid-state NMR and computational chemistry study of mononucleotides adsorbed to alumina. Langmuir 22:9281–9286
Smith JAS (1971) Nuclear quadrupole resonance spectroscopy. J Chem Educ 48:39–41
Garroway AN (2003) Appendix K: Nuclear quadrupole resonance, by, Naval Research Laboratory. In: Jacqueline MacDonald JR (ed) Lockwood: Alternatives for landmine detection. Report MR-1608. Rand Corporation
Poleshchuck OK, Kalinna EL, Latosinska JN, Koput J (2001). J Mol Struct Theochem 547:233–243
Young HA, Freedman RD (2012) Sears and Zemansky’s University Physics with Modern Physics13th edn. Addison-Wesley, Boston, p 754
Cortés-Arriagada D, Villegas-Escobar N, Ortega DE (2018) Fe-doped graphene nanosheet as an adsorption platform of harmful gas molecules (CO, CO2, SO2 and H2S), and the co-adsorption in O2 environments. Appl Surf Sci 427, Part B:227–236. https://doi.org/10.1016/j.apsusc.2017.08.216
Tahan A, Mollaamin F, Monajjemi M (2009) Thermochemistry and NBO analysis of peptide bond: Investigation of basis sets and binding energy. Russ J Phys Chem A 83:587–597. https://doi.org/10.1134/S003602440904013X
Kohn W, Becke AD, Parr RG (1996) Density functional theory of electronic structure. J Phys Chem 100:12974–12980. https://doi.org/10.1021/jp960669l
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc. 105:7512–7516. https://doi.org/10.1021/ja00364a005
Politzer P, Abu–Awwad FA (1998) Comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor Chem Acc 99:83–87. https://doi.org/10.1007/s002140050307
Aihara J (1999) Reduced HOMO−LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A 103(37):7487–7495. https://doi.org/10.1021/jp990092i
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrometric identification of organic compounds5th edn. John Wiley & Sons, Inc., New York
Acknowledgements
In successfully completing this paper and its research, the authors are grateful to Kastamonu University for their support through the library, the laboratory, and scientific websites.
Author information
Authors and Affiliations
Contributions
Fatemeh Mollaamin: conceptualization and idea, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, visualization, supervision, project administration. Majid Monajjemi: methodology, software, formal analysis, investigation, data curation, writing—review and editing, visualization, resources.
Corresponding author
Ethics declarations
Ethics approval
The authors consent to participate and publish the data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mollaamin, F., Monajjemi, M. Transition metal (X = Mn, Fe, Co, Ni, Cu, Zn)-doped graphene as gas sensor for CO2 and NO2 detection: a molecular modeling framework by DFT perspective. J Mol Model 29, 119 (2023). https://doi.org/10.1007/s00894-023-05526-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05526-3