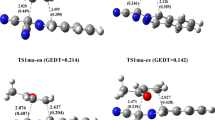
Abstract
The present computational study using B3LYP functional and 6-31+G(d) basis set has been accomplished to investigate the mechanism of the inverse demand Diels-Alder reaction between pyridyl imine and propene. The highly charged dicationic superelectrophilic diene with exceptionally low-lying LUMO makes the cycloaddition reaction with propene more favorable by significantly lowering the activation energy. The Wiberg bond indices are calculated in accordance with the formation and breaking processes of bonds. The synchronicity concept is also utilized to explain the global nature of the reaction. A potential outcome of this investigation is the utilization of propene as a C2 building block in the industry.
Graphical abstract






Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the manuscript and its Supplementary Information.
References
Johannsenand M, Jørgensen KA (1998) Allylic amination. Chem Rev 98:1689–1708. https://doi.org/10.1021/CR970343O
Müller TE, Beller M (1998) Metal-initiated amination of alkenes and alkynes. Chem Rev 98:675–703. https://doi.org/10.1021/CR960433D
Halfen J (2005) Recent advances in metal-mediated carbon-nitrogen bond formation reactions: aziridination and amidation. Curr Org Chem 9:657–669. https://doi.org/10.2174/1385272053765024
Liang C, Collet F, Robert-Peillard F et al (2008) Toward a synthetically useful stereoselective C-H amination of hydrocarbons. J Am Chem Soc 130:343–350. https://doi.org/10.1021/JA076519D
Wang H, Wang Y, Peng C et al (2010) A direct intramolecular C−H amination reaction cocatalyzed by copper(II) and iron(III) as part of an efficient route for the synthesis of pyrido[1,2- a ]benzimidazoles from N -aryl-2-aminopyridines. J Am Chem Soc 132:13217–13219. https://doi.org/10.1021/ja1067993
Masters K-S, Rauws TRM, Yadav AK et al (2011) On the importance of an acid additive in the synthesis of pyrido[12,-a]benzimidazoles by direct copper-catalyzed amination. Chem–A Eur J 17:6315–6320. https://doi.org/10.1002/CHEM.201100574
Maes J, Rauws TRM, Maes BUW (2013) Synthesis of C8–N9 annulated purines by iron-catalyzed C-H amination. Chemistry 19:9137–9141. https://doi.org/10.1002/CHEM.201301248
Vuong H, Dash BP, Nilsson Lill SO, Klumpp DA (2018) Diels-Alder reactions with ethylene and superelectrophiles. Org Lett 20:1849–1852. https://doi.org/10.1021/ACS.ORGLETT.8B00367
Olah GA, Germain A, Lin HC, Forsyth DA (2002) Electrophilic reactions at single bonds. XVIII. Indication of protosolvated de facto substituting agents in the reactions of alkanes with acetylium and nitronium ions in superacidic media. J Am Chem Soc 97:2928–2929. https://doi.org/10.1021/JA00843A067
Olah GA, Klumpp DA (2008) Superelectrophiles and their chemistry. Wiley-Interscience, p 301
Suzuki T, Ohwada T, Shudo K (1997) Superacid-catalyzed electrocyclization of 1-phenyl-2-propen-1-ones to 1- indanones. Kinetic and theoretical studies of electrocyclization of oxonium- carbenium dications. J Am Chem Soc 119:6774–6780. https://doi.org/10.1021/JA971100G
Olah GA, Koltunov KY, Prakash GS, Rasul G (2004) Reactions of 2-, 3-, and 4-quinolinols with cyclohexane and benzene in superacids. Heterocycles 62:757. https://doi.org/10.3987/com-03-s(p)74
Domingo LR, Ríos-Gutiérrez M, Emamian S (2016) Understanding the stereoselectivity in Brønsted acid catalysed Povarov reactions generating cis/trans CF3-substituted tetrahydroquinolines: a DFT study. RSC Adv 6:17064–17073. https://doi.org/10.1039/c5ra27650k
Ríos-Gutiérrez M, Layeb H, Domingo LR (2015) A DFT study of the mechanism of Brønsted acid catalysed Povarov reactions. Tetrahedron 71:9339–9345. https://doi.org/10.1016/J.TET.2015.10.012
Kouznetsov VV (2009) Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 65:2721–2750. https://doi.org/10.1016/J.TET.2008.12.059
Morizur J-P, Mercier J, Sarraf M (1982) 2-substituted-2,3-dihydro-4H-pyrans: competition between ‘retro Diels-Alder’ fragmentation and substituent loss. Org Mass Spectrom 17:327–330. https://doi.org/10.1002/OMS.1210170708
Derrick PJ, Åsbrink L, Edqvist O et al (1971) Rydberg series in small molecules: X. Photoelectron spectroscopy and electronic structure of furan. Int J Mass Spectrom Ion Phys 6:161–175. https://doi.org/10.1016/0020-7381(71)80001-3
Hunter EPL, Lias SG, Hunter EPL, Lias SG (1998) Evaluated gas phase basicities and proton affinities of molecules: an update. JPCRD 27:413–656. https://doi.org/10.1063/1.556018
Dewar MJS, Haselbach E, Worley SD (1970) Calculated and observed ionization potentials of unsaturated polycyclic hydrocarbons; calculated heats of formation by several semiempirical s. c. f. m.o. methods. Proc R Soc London A Math Phys Sci 315:431–442. https://doi.org/10.1098/RSPA.1970.0053
Watanabe K, Nakayama T, Mottl J (1962) Ionization potentials of some molecules. J Quant Spectrosc Radiat Transf 2:369–382. https://doi.org/10.1016/0022-4073(62)90023-7
Bock H, Seidl H (1968) d-orbitaleffekte in silizium-substituierten π-elektronensystemen VI. Spektroskopische untersuchungen an alkyl- und silyläthylenen. J Organomet Chem 13:87–102. https://doi.org/10.1016/S0022-328X(00)88859-2
Traeger JC (1984) A study of the allyl cation thermochemistry by photoionization mass spectrometry. Int J Mass Spectrom Ion Process 58:259–271. https://doi.org/10.1016/0168-1176(84)80034-8
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, et al (2016) Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT
Lee C, Yang W, Parr RG (1988) Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B: Condens Matter Mater Phys 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Becke AD (1993) Densityfunctional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Petersson GA, Bennett A, Tensfeldt TG et al (1988) A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J Chem Phys 89:2193–2218. https://doi.org/10.1063/1.455064
Petersson GA, Al-Laham MA (1998) A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J Chem Phys 94:6081. https://doi.org/10.1063/1.460447
Frisch MJ, Pople JA, Binkley JS (1998) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265. https://doi.org/10.1063/1.447079
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) Using redundant internal coordinates to optimize equilibrium geometries and transition states. J Comput Chem 17:49–56
Gonzalez C, Bernhard Schlegel H (1998) An improved algorithm for reaction path following. J Chem Phys 90:2154. https://doi.org/10.1063/1.456010
Fukui K (1981) The path of chemical reactions - the IRC approach. Acc Chem Res 14:363–368. https://doi.org/10.1021/AR00072A001/ASSET/AR00072A001.FP.PNG_V03
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/JP9716997
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4%3c404::AID-JCC3%3e3.0.CO;2-W
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681. https://doi.org/10.1002/JCC.10189
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1998) NBO Version 3.1, TCI, University of Wisconsin, Madison.
Nandi S, Monesi A, Drgan V et al (2013) Quantitative structure-activation barrier relationship modeling for Diels-Alder ligations utilizing quantum chemical structural descriptors. Chem Cent J 7:1–13. https://doi.org/10.1186/1752-153X-7-171/FIGURES/5
Klumpp DA (2008) Superelectrophiles: charge–charge repulsive effects. Chem - A Eur J 14:2004–2015. https://doi.org/10.1002/CHEM.200701470
Badenhoop JK, Weinhold F (1999) Natural steric analysis of internal rotation barriers. Int J Quantum Chem 72:269. https://doi.org/10.1002/(SICI)1097-461X
Lendvay G (2002) Bond orders from ab initio calculations and a test of the principle of bond order conservation. J Phys Chem 93:4422–4429. https://doi.org/10.1021/J100348A011
Shiroudi A, Zahedi E (2012) Theoretical study and NBO analysis of the kinetics and mechanism of the gas phase elimination reactions of 2-chloroethylsilane and derivatives. Prog React Kinet Mech 37:76–89. https://doi.org/10.3184/146867812X13242290723354
Reed AE, Curtiss LA, Weinhold F (2002) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/CR00088A005
Reed AE, Weinstock RB, Weinhold F (1998) Natural population analysis. J Chem Phys 83:735. https://doi.org/10.1063/1.449486
Chuang C-H, Lien M-H (2004) Computational study on the effects of substituents and functional groups in the isomerization of 1- and 2-substituted propenes, acetaldimines, and aldehydes. European J Org Chem 2004:1432–1443. https://doi.org/10.1002/EJOC.200300666
Acknowledgements
Authors acknowledge the necessary research facilities provided by the Department of Chemistry, Indian Institute of Engineering Science and Technology, Shibpur.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, data collection, and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, S., Roy, R.S. & Nandi, P.K. Unveiling the theoretical aspects of superelectrophilic activation in an inverse demand Diels-Alder reaction. J Mol Model 29, 89 (2023). https://doi.org/10.1007/s00894-023-05495-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05495-7