Abstract
Context
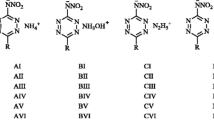
In modern searches for the structure of high-energy-density compounds with high operational, detonation, and physicochemical characteristics, a special place belongs to salts, which have a number of significant advantages over neutral compounds. The development of this area of HEDM is hampered by the lack of effective calculation schemes for estimating the enthalpy of formation DHf0 of salts, as a key parameter in assessing the prospects for their use. Based on the author’s method (MICCM), which is superior in accuracy to currently available calculation methods, the enthalpies of formation of various salts of nitrates and perchlorates for a promising class of high-energy amino-1,2,4-triazoles are calculated and the accuracy of calculations is estimated by other methods. Relationships between the thermochemical characteristics of salts depending on various cations are considered. Among the considered compounds, calculations of the enthalpies of salts of three amino-1,2,4-triazoles showed a significant discrepancy with the experimental data.
Methods
Calculations DHf0of salts were performed using three methods: volume-base thermodynamic (Jenkins/Bartlett method), the method of adding of ions contributions (MAIC, Matyushin’s method), and the method of ions and cocrystals contribution mixing (MICCM, Khakimov’s method). Calculations by the MICCM method were carried out on the basis of quantum chemistry methods (when estimating the enthalpies of formation in the gas phase) and the method of atom-atom potentials (AAP) when calculating the enthalpy of sublimation of salts. We have optimized all the structures in the gas phase using the Becke three hybrid exchange and Lee-Yang-Parr correlation functional with Grimme’s dispersion correction, B3LYP-D2, and aug-cc-pVDZ basis set using the Gaussian16 software. The AAP calculations were performed using the FitMEP software packages (for adjusting the charges of the molecular electrostatic potential) and PMC (for the procedure for constructing crystal packings and searching for optimal ones).




Similar content being viewed by others
References
Yao W, Xue Y, Quia L, Yang H, Cheng G (2021) Combination of 1,2,3-triazole and 1,2,4-triazole frameworks for new high-energy and low-sensitivity compounds. Energetic Materials Frontiers 2:131–138
Rudakov GF, Sinditskii VP, Andreeva IA, Botnikova AI, Veselkina PR, Konstakyan SK, Yudin NV, Serushkin VV, Cherkaev GV, Dorofeeva OV (2022) Energetic compounds based on a new fused bis[1,2,4]triazolo[1,5-b;5’,1’-f]-1,2,4,5-tetrazine. Chem Eng J 450:138073
Mathpati RS, Yadav AK, Ghule VD, Dharavath S (2022) Potential energetic salts of 5,5’-methylenedi(4H–1,2,4-triazole-3,4-diamine) cation: synthesis, characterization and detonation performance. Energetic Materials Frontiers 3:90–96
Chaplygin DA, Larin AA, Muravyev NV, Meerov DB, Kosareva EK, Kisilev VG, Pivkina AN, Ananyev IV, Fershtat LL (2021) Nitrogen-rich metal-free salts: a new look at the 5-(trinitromethyl)tetrazolate anion as an energetic moiety. Dalton Trans 50:13778–13785
Bayat Y, Taheripouya G (2018) Synthesis of novel energetic N-(1-carboxymethyl-1H-tetrazole-5-yl)-hydrazinium salts. Cent Eur J Energ Mater 15:420–434
Bauer L, Benz M, Klapötke TM, Pignot C, Stierstorfer J (2022) Combining the most suitable energetic tetrazole and triazole moieties: synthesis and characterization of 5-(1-hydroxy-3-nitro-1,2,4-triazol-5-yl)-1-hydroxy-tetrazole and its nitrogen-rich ionic derivatives. Mater Adv 3:3945–3951
Gettings ML, Byrd EFC, Zeller M, Piercey D (2021) Methyl sydnone imine and its energetic salts. New J Chem 45:2228–2236
Mallouk TE, Rosenthal GL, Muller G, Busasco R, Bartlett N (1984) Fluoride ion affinities of germanium tetrafluoride and boron trifluoride from thermodynamic and structural data for (SF3)2GeF6, ClO2GeF5, and ClO2BF4. Inorg Chem 23:3167–3173
Jenkins HDB, Tudela D, Glasser L (2002) Lattice potential energy estimation for complex ionic salts from density measurements. Inorg Chem 41:2364–2367
Matyushin YuN, Kon´kova TS, (2014) The method for assessment of thermochemical properties for salt compounds. Gorenie i Vzryv [Combustion and Explosion] 7:277–287
Khakimov DV, Pivina TS (2022) A new method for predicting the enthalpy of salt formation. J Phys Chem A 126:5207–5214
Klapotke TM (2012) Computational methods, in: Chemistry of high energy materials, Walter de Gruyter, Berlin–Boston, 89.
Montgomery JJA, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JA (1998) Gaussian-3 (G3) theory for molecules containing first and second-row atoms. J Phys Chem 109:7764–7776
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, revision D.01, Gaussian, Inc., Wallingford CT.
Dzyabchenko AV (2008) A multipole approximation of the electrostatic potential of molecules. Russ J Phys Chem A 82:758–766
Dzyabchenko AV (2008) From molecule to solid: the prediction of organic crystal structures. Russ J Phys Chem A 82:1663–1671
Khakimov DV, Dzyabchenko AV, Pivina TS (2019) Crystal structure prediction of bifurazano[3,4-b:3’,4’-f]furoxano[3",4"-d]-oxacycloheptatriene (BFFO) in the experimentally known monohydrated and proposed anhydrous forms. Propellants, Explos, Pyrotech 44:1528–1534
Khakimov DV, Zelenov VP, Baraboshkin NM, Pivina TS (2019) The unusual combination of beauty and power of furoxano-1,2,3,4-tetrazine 1,3-dioxides: a theoretical study of crystal structures. J Mol Model 25:107
Khakimov DV, Fershtat LL, Pivina TS, Makhova NN (2021) Nitrodiaziridines: unattainable yet, but desired energetic materials. J Phys Chem A 125:3920–3927
Momany FA, Carruthers LM, McGuire RF, Scheraga HA (1974) Intermolecular potentials from crystal data. III. Determination of empirical potentials and application to the packing configurations and lattice energies in crystals of hydrocarbons, carboxylic acids, amines, and amides. J Phys Chem 78:1595–1620
Cruz-Cabeza AJ, Pidcock E, Day GM, Motherwell WDS, Jones W (2007) Space group selection for crystal structure prediction of solvates. CrystEngComm 9:556–560
Chase MW (1998) NIST-JANAF thermochemical tables, American Institute of Physics, 4th Edition 1–1952.
Kon´kova TS, Matyushin YuN, Miroshnichenko EA, Vorob´ev AB, (2009) Thermochemical properties of dinitramidic acid salts. Russ Chem Bull 58:2020–2027
Gilliland AA (1962) Heat of formation of nitronium perchlorate. Journal of Research of the National Bureau of Standards-A. Physics and Chemistry 66:447–449
Markowitz MM, Harris RF, Stewart H Jr (1959) The heat of formation of anhydrous lithium perchlorate. J Phys Chem 63:1325–1326
Rafeev VA, Rubtsov YI (1993) Kinetics and mechanism of thermal decomposition of hydroxylammonium nitrate. Russ Chem Bull 42:1811–1815
Wingeborg N, Latypov NV (2003) Triaminoguanidine dinitramide, TAGDN: synthesis and characterization. Propellants, Explos. Pyrotech 28:314–318
Xue H, Arritt SW, Twamley B, Shreeve JM (2004) Energetic salts from N-aminoazoles. Inorg Chem 43:7972–7977
Shreeve JM (2007) Ionic liquids as energetic materials. Report AFRL-SR-AR-TR-07–0094.
Pokon EK, Liptak MD, Feldgus S, Shields GC (2001) Comparison of CBS-QB3, CBS-APNO, and G3 predictions of gas phase deprotonation data. J Phys Chem A 105:10483–11087
Williams MM, McEwan WS, Henry RA (1957) The heat of combustion of substituted triazoles, tetrazoles and related high nitrogen compounds. J Phys Chem 61:261–267
Kon´kova TS, Matyushin YuN, Miroshnichenko EA, Makhov MN, Vorob´ev AB, Inozemtsev AV, (2018) Energetic properties of derivatives of 1,2,4-triazole. Gorenie i Vzryv [Combustion and Explosion] 11:90–99
Schaller U, Keicher T, Weiser V, Krause H, Schlechtriem S (2010) Characterization and combustion of triazolium based salts. In Insensitive munitions and energetic materials technology symposium, Munich, Germany, October 11–14.
Zhang Z, Zhang J, Gozin M (2018) Nitrogen-rich salts based on 1,1’-dihydroxy-5,5’-azobistetrazole: a new family of energetic materials with promising properties. ChemistrySelect 3:3463–3473
Author information
Authors and Affiliations
Contributions
DK did the calculations and came up with the concept of the work and wrote the original text. TP reviewed the work, wrote the conclusions, and participated in the discussion of the work.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khakimov, D.V., Pivina, T.S. Is everything correct? The formation enthalpy estimation and data revision of nitrate and perchlorate salts. J Mol Model 29, 75 (2023). https://doi.org/10.1007/s00894-023-05477-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05477-9