Abstract
Introduction
The use of the Cannabis sativa plant by man has been common for centuries due to its numerous therapeutic properties resulting from the compounds present in it, called cannabinoids. However, the use of these compounds as drugs is still limited due to the psychotropic effects caused by them. The proteins that act as receptors of cannabinoid compounds were identified and characterized, being called CB1 and CB2 receptors. There is a series of 50 cannabinoid compounds that was studied through quantum and chemometric methods in order to obtain a mathematical model that could relate the structure of these compounds to their psychotropic activity. That model proved to be effective by predicting the psychoactivity of the 50 compounds from the series and elucidating relevant characteristics that imply in psychoactivity. However, most of these 50 compounds do not have experimental data of biological activity with CB1 and CB2 receptors.
Objectives
This study aims to generate QSAR models in order to predict the biological activity of the 50 cannabinoid compounds and then relate the predicted biological activity values to the already known psychoactivity.
Methods
Another series of cannabinoid compounds was selected to generate and validate QSAR models, aiming to predict the biological activity of the 50 cannabinoid compounds with both CB1 and CB2 receptors.
Results
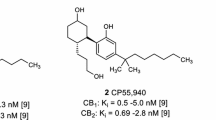
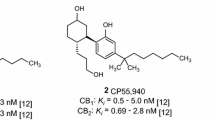
The PLS-CB1 and PLS-CB2 QSAR models were generated and validated in this work, proving to be highly predictive, and the biological activities (pK ) of the 50 cannabinoid compounds were predicted by them. It is important to highlight compounds Ic14, Ic18, and Ic19 (psychotropic inactive) which presented higher predicted pK values than the main cannabinoid compounds (Δ9-THC and Δ8-THC). Also, compound Ic21 stood out as the highest value of the predicted biological activities in the interaction with the CB2 receptor.
Conclusion
The generated PLS models and the predicted pKi values of the 50 cannabinoid compounds can provide valuable information in the drug design of new cannabinoid compounds that can interact with CB1 and CB2 receptors in a therapeutic way with no psychotropic effects.
























Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Zuardi AW (2006) History of cannabis as a medicine: a review. Braz J Psychiatry 28:153–157. https://doi.org/10.1590/S1516-44462006000200015
Pertwee RG (2006) Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 147:163–171. https://doi.org/10.1038/sj.bjp.0706406
Brooks JW (2002) Cannabinoids and analgesia. Trends Anaesth Crit Care 13:215–220. https://doi.org/10.1054/cacc.2002.0406
Reuter SE, Martin JH (2016) Pharmacokinetics of cannabis in cancer-cachexia anorexia syndrome. Clin Pharmacokinet 55:807–812. https://doi.org/10.1007/s40262-015-0363-2
Todaro B (2012) Cannabinoids in the treatment of chemothrapy-induced nausea and vomiting. J Natl Compr Canc Netw 10:487–492. https://doi.org/10.6004/jnccn.2012.0048
Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Hagen U, Schnelle M, Reif M (2004) Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler 10:417–424. https://doi.org/10.1191/1352458504ms1048oa
El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, Khan I, Elsohly M, Ross S (2010) Antidepressant-like effect of Δ9-tetrahidrocanabinol and other canabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav 95:434–442. https://doi.org/10.1016/j.pbb.2010.03.004
Atakan Z (2012) Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol 2:241–254. https://doi.org/10.1177/2045125312457586
Mechoulam R, Burstein SH (1973) Marijuana: chemistry, pharmacology, metabolism and clinical effects. Academic Press, New York
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Boner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564. https://doi.org/10.1038/346561a0
Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessègue B, Bonnin-Cabanne O, Le Fur G, Caput D, Ferrara P (1996) Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim Biophys Acta Gene Regul Mech 1307:132–136. https://doi.org/10.1016/0167-4781(96)00047-4
Honório KM, Silva ABF (2005) A study on the influence of molecular properties in the psychoactivity of cannabinoid compounds. J Mol Model 11:200–209. https://doi.org/10.1007/s00894-005-0243-z
Honório KM, Lima EF, Quiles MG, Romero RA, Molfetta FA, Da Silva ABF (2010) Artificial neural networks and the study of the psychoactivity of cannabinoid compounds. Chem Biol Drug Des 75:632–640. https://doi.org/10.1111/j.1747-0285.2010.00966.x
MECHOULAM R, (1973) Marijuana: chemistry, pharmacology, metabolism and clinical effects. Academic Press, New York
Petersen RC (1980) Marijuana research findings: 1980. NIDA Res Monogr 31:1–225
Mechoulam R (1970) Marihuana chemistry. Science 168:1159–1166
da Silva ABF, Trsic M (1995) Theoretical and conformational studies of a series of cannabinoids. J Mol Struct 356:247–256
Razdan RK (1986) Structure-activity relationships in cannabinoids. Pharmacol Rev 38:75–149
Roy K, Das RN (2014) A review on principles, theory and practices of 2D-QSAR. Curr Drug Metab 15:346–379. https://doi.org/10.2174/1389200215666140908102230
Davies M, Nowotka M, Papadatos G, Dedman N, Gaulton A, Atkinson F, Bellis L, Overington JP (2015) ChEMBL web services: streamlining access to drug discovery data and utilities. Nucleic Acids Res 43:W612–W620. https://doi.org/10.1093/nar%2Fgkv352
Mendez D, Gaulton A, Bento AP, Chambers J, Veij MD, Felix E, Margarinos MP, Mosquera JF, Mutowo P, Nowotka M, Gordillo-Marañon M, Hunter F, Junco L, Mugumbate G, Rodriguez-Lopes M, Atkinson F, Bosc N, Radoux CJ, Segura-Cabrera A, Hersey A, Leach AR (2019) ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res 47:D930–D940. https://doi.org/10.1093/nar/gky1075
Gaulton A, Hersey A, Nowotka M, Bento AP, Chambers J, Mendez D, Mutowo P, Atkinson F, Bellis LJ, Cibrián-Uhalte E, Davies M, Dedman N, Karlsson A, Margariños MP, Overington JP, Papadatos G, Smit I, Leach AR (2017) The ChEMBL database in 2017. Nucleic Acids Res 45:D945–D954. https://doi.org/10.1093/nar/gkw1074
Bento AP, Gaulton A, Hersey A, Bellis LJ, Chambers J, Davies M, Krüger FA, Light Y, Mak L, Mcglinchey S, Nowotka M, Papadatos G, Santos R, Overington JP (2014) The ChEMBL bioactivity database: an update. Nucleic Acids Res 42:D1083–D1090. https://doi.org/10.1093/nar/gkt1031
Frisch MJ, Trucks GW, Schlegel HB et al (2016) Gaussian 09, Revision A.02 Gaussian Inc, Wallingford CT
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37, 785. https://doi.org/10.1103/PhysRevB.37.785
Poople JA, Seeger R, Binkley JS, Krishnan R (1980) Selfconsistent molecular orbital method.s XX. A basis set for correlated wave functions. J Chem Phys 72:650. https://doi.org/10.1063/1.1677527
Tetko IV, Gasteiger J, Todeschini R et al (2005) Virtual computational chemistry laboratory - design and description. J Comput Aid Mol Des 19:453–463. https://doi.org/10.1007/s10822-005-8694-y
Asuero AG, Sayago A, González AG (2007) The correlation coeficcient: an overview. Crit Rev Anal Chem 36:41–59. https://doi.org/10.1080/10408340500526766
Kumar M, Husian M (2010) Genetic algorithm: review and application. Int J Inf Tech Knowl Manag 2:451–454
De Oliveira DB, Gaudio AC (2000) BuildQSAR: a new computer program for QSAR analysis. Quant Struct -Act Relationships 19:599–601. https://doi.org/10.1002/1521-3838%28200012%2919%3A6%3C599%3A%3AAID-QSAR599%3E3.0.CO%3B2-B
Pirouette 3.11. (2002) Infometrix Inc, Woodinville
Martins JPA, Ferreira MMC (2013) QSAR modeling: a new open source computational package to generate and validate QSAR models. Quim Nova 36:554–560. https://doi.org/10.1590/S0100-40422013000400013
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20:269–276. https://doi.org/10.1016/S1093-3263(01)00123-1
Kiralj R, Ferreira MMC (2009) Basic validation procedures for regression models in QSAR and QSPR studies: theory and application. J Braz Chem Soc 20:770–787. https://doi.org/10.1590/S0103-50532009000400021
Karelson M, Lobanov VS, Katrizky AR (1996) Quantum-chemical descriptors in QSAR/QSPR studies. Chem Rev 96:1027–1043. https://doi.org/10.1021/cr950202r
Todeschini R, Consonni V (2000) Handbook of molecular descriptors, Wiley–VCH. Weinheim. https://doi.org/10.1002/9783527613106
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance Code 001), FAPESP (2016/10118–0, 2018/06680–7, 2016/24524–7, 2017/10118–0), and CNPq. Our research was carried out using the computational resources of the Center for Mathematical Sciences Applied to Industry (CeMEAI) funded by FAPESP (grant 2013/07375–0).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Laise P. A. Chiari and Aldineia P. da Silva. The first draft of the manuscript was written by Laise P. A. Chiari, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection XXI - Brazilian Symposium of Theoretical Chemistry (SBQT2021)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiari, L.P.A., da Silva, A.P., Honório, K.M. et al. A PLS study on the psychotropic activity for a series of cannabinoid compounds. J Mol Model 29, 46 (2023). https://doi.org/10.1007/s00894-023-05443-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05443-5