Abstract
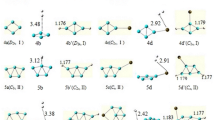
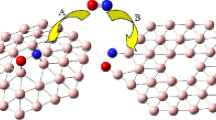
The equilibrium structures of BeO clusters and Be,Ti-decorated boranes were computed with the ωB97X-D method and the 6-31G + (2d,2p) and aug-cc-pVTZ basis sets to study their intermolecular interactions with hydrogen molecules. Thermochemical and molecular properties such as the harmonic vibrational frequency, dipole and quadrupole moments, and atomic charges are employed to understand the attractive interactions that control the adsorption process. Comparison of molecular properties and atomic charges of the studied compounds before and after H2 molecule adsorption shows that most of the interactions among the BeO clusters and boranes with H2 molecules constitute a combination of dispersion, electrostatic, and weak charge transfer interactions. Calculated values of Hirschfeld atomic charges and ΔEe (in parenthesis) (BeO)4.8H2 (0.028 e and –2.0 kcal.mol-1), (BeO)2.12H2 (0.030 e and –2.8 kcal.mol-1), B6Ti3.10H2 (0.045 e and –15.4 kcal.mol-1), and B6Ti3+.10H2 (0.058 e and –15.3 kcal.mol-1) show qualitative correlation between hydrogen atomic charges and electronic energy of hydrogen interaction. The ωB97X-D/6–31 + G(2d,2p) values of Gibbs free energy at 298.15 K for (BeO)4.8H2 B2H4Ti.4H2 and B6Ti3.10H2 clusters are equal to –5.0, –4.9, and –5.1 kcal.mol-1, respectively, which are within the range of energy parameters of materials that could be employed in hydrogen storage tanks for light vehicles.
Graphical Abstract





Similar content being viewed by others
Data availability
All relevant data are available in the electronic supplementary information.
Code availability
Gaussian 09 Revision A.02 is used for all the calculations in this work.
References
Report of IPCC 2022. Access on September 12, 2022: https://www.ipcc.ch/report/ar6/wg2/.
Chomsky N, Pollin R, Polychroniou CJ (2020) Climate crisis and global green deal. Verso, London
Clean Hydrogen Partnership through the Horizon Europe Programme. Access on November 4, 2022. https://www.clean-hydrogen.europa.eu/media/news/accelerating-hydrogen-economy-2022-05-18_en
DOE technical targets for onboard hydrogen storage for light duty vehicles. https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles
Suh MP, Park HJ, Prasad TK, Lim D-W (2012) Hydrogen storage in metal-organic frameworks energy. Chem Rev 112:782. https://doi.org/10.1021/cr200274s
Oliveira ACM, Pavão AC (2018) Theoretical study of hydrogen storage in metal hydrides. J Mol Model 24:127–134. https://doi.org/10.1007/s00894-018-3661-4
Tsivion E, Long JR, Head-Gordon M (2014) Hydrogen physisorption on metal-organic framework linkers and metalated linkers: a computational study of that control binding strength. Am Chem Soc 136(51):17827–17835. https://doi.org/10.1021/ja5101323
Jensen J, Introduction to computational chemistry (2007), John Wiley & Sons, West Sussex, England, 2a Edition, 34-50
Kubas GJ (2007) Fundamentals of H2 binding and reactivity on transition metals underlying hydrogenase function and H2 production and storage. Chem Rev 107:4152–4205. https://doi.org/10.1021/cr050197j
Maia RA, Oliveira FL, Ritleng V, Wang Q, Benoît L, Esteves PM (2021) CO2 Capture by hydroxylated azine-based covalent organic frameworks. Chem A Eur J 27:8048–8055. https://doi.org/10.1002/chem.202100478
Kumar S, Kumar TJD (2017) Electronic structure calculations of hydrogen storage in lithium-decorated metal-graphyne framework Appl Mater. Interfaces 9(34):28659–28666. https://doi.org/10.1021/acsami.7b09893
Liu Z, Liu S, Suleyman ER (2019) Hydrogen storage properties of Li-decorated B2S monolayers: a DFT study. Int J Hydrogen Energy 44:16803–16810. https://doi.org/10.1016/j.ijhydene.2019.04.234
Shind R, Tayade M (2014) Remarkable hydrogen storage on beryllium oxide clusters: first-principles calculations. J Phys Chem C 118(31):17200–17204. https://doi.org/10.1021/jp4109943
Roberto-Neto O, Carvalho EFV (2020) A DFT and wave function theory study of hydrogen adsorption on small beryllium oxide clusters. Theor Chem Accounts 139:93–103. https://doi.org/10.1007/s00214-020-02605-z
Konda R, Titus E, Chaudhari A (2018) Adsorption of molecular hydrogen on inorganometallic complexes B2H4M (M = Li, Be, Sc, Ti, V). Struct Chem 29(6):1593–1599. https://doi.org/10.1007/s11224-018-1128-y
Guo C, Wang C (2018) Computational investigation of hydrogen storage on B6Ti3+. Int J Hydrog Energy 43:1658–1666. https://doi.org/10.1016/j.ijhydene.2017.11.161
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Physics 44:6615–6620. https://doi.org/10.1039/B810189B
Grimme S (2011) Density functional theory with London dispersion corrections. Adv Rev 1:211–227. https://doi.org/10.1039/B810189B
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I The atoms boron through neon and hydrogen J Chem Phys 90:1007–1018. https://doi.org/10.1063/1.456153
Hirschfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138. https://doi.org/10.1007/BF00549096
Guerra CF, Handgraaf J-W, Baerends EJ, Bickelhaupt FM (2003) Voronoi deformation density (VDD) charges: assessment of the Mulliken Bader, Hirshfeld, and VDD Methods for Charge Analysis. J Comput Chem 25:189–210. https://doi.org/10.1002/jcc.10351
Frish MJ et al (2009) GAUSSIAN 09 revision B.01, Gaussian Inc, Wallingford. CT
Hobza P, Zharadnik R, Müller-Dethlefs K (2006) The world of non-covalent interactions: Collect. Czech Chem Commun 71:443–531. https://doi.org/10.1135/cccc20060443
Boeyens JCA (2008) The periodic electronegativity table. Zeitschrift fur Naturforschung B 63:199–209. https://doi.org/10.1515/znb-2008-0214
Areán CO, Bonelli B, Delgado MR, Garrone E (2009) Hydrogen storage via physisorption: the combined role of adsorption enthalpy and entropy. Turk J Chem 33:599–606. https://doi.org/10.3906/kim-0812-22
Pearson’s Correlation Coefficient (2008). In: Kirch, W. (eds) Encyclopedia of Public Health. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-5614-7_2569.
Peterson KA, Feller D, Dixon DA (2012) Chemical accuracy in ab initio thermochemistry and spectroscopy: current strategies and future challenges. Theoret Chem Acc 131(1):1–20
Kapelewski MT, Ručevski T, Tarver JD, Jiang HZH, Hurst KE, Parilla PA, Ayala A, Gennett T, FitzGerald SA, Brown CM, Long JR (2018) Record high hydrogen storage capacity in the metal-organic framework Ni2 (m-dobdc) at near-ambient temperatures. Chem Mater 30:8179. https://doi.org/10.1021/acs.chemmater.8b03276
Funding
We acknowledge the research support of the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) under grant no. 06414/22 and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under grant no. 480119/2012–0.
Author information
Authors and Affiliations
Contributions
All authors gave equivalent contributions in the planning, development of calculations, analysis of data, and writing of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection XXI - Brazilian Symposium of Theoretical Chemistry (SBQT2021).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vasconcellos, L.C., de Carvalho, E.F.V. & Roberto-Neto, O. Hydrogen physisorption on the (BeO)n, B2H4(Be,Ti), and B6Ti3 metal clusters: a computational study of energies and atomic charges. J Mol Model 29, 48 (2023). https://doi.org/10.1007/s00894-022-05432-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05432-0