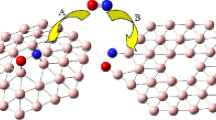
Abstract
In the last centuries, human society has strongly modified the biosphere of the planet through consumption of its resources on a highly increasing scale but without planning, causing pollution in various environments through chemical dumping, and the extensive use of fossil fuels in industrialization and transportation, for example. In this last aspect, the use of less polluting alternative fuels for vehicular transport based on the consumption of electricity and hydrogen gas has been suggested. Experimental and theoretical studies are being made to increase the hydrogen storage capacity using porous carbon materials, nanoparticles, and metallic clusters. Most of the electronic and structure calculations of such materials have been carried out with density functional theory (DFT) without gauging the accuracy of the employed DFTs. In this work, we have employed various DFTs to calculate hydrogen adsorption energies of small clusters of BeO and compare with wave function theory methods, in order to evaluate the relative accuracy of these methodologies, since experimental values are not usually available. The hydrogen storage capability of small clusters of (BeO)n (n = 2–7) is studied with various density functionals, MP2, and CCSD(T) methods and benchmarked with the complete basis set (CBS) limit approach. Calculated properties are the equilibrium geometries of isolated and adsorbed clusters with H2, electronic adsorption energy, enthalpy, and Gibbs free energy. Calculations with the CBS method are employed to gauge the accuracy of various methods in calculating structures and cohesive energies of small beryllium clusters. The smallest values of mean unsigned error of the cohesive energies (Eb) calculated with the ωB97X-D, TPPSh, and M06-L DFTs are equal to 0.05, 0.06, and 0.07 eV using as reference the CBSD-T.

Similar content being viewed by others
References
Steffen W, Grinevald J, Crutzen P, McNeill J (2011) The Anthropocene: conceptual and historical perspectives. Philos Trans R Soc A 369:842–867. https://doi.org/10.1098/rsta.2010.0327
Ophuls W (2013) Plato’s revenge: politics in the age of ecology. MIT Press, Cambridge, p 25
Serres M (2015) Time of crisis: what the financial crisis revealed and how to reinvent our lives and future. Bloomsbury Academic, New York, p p10
Steinfeld A (2002) Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int J Hydrog Energy 27:611–619. https://doi.org/10.1016/S0360-3199(01)00177-X
Kumar S, Jain A, Ichikawa T, Kojima Y, Dey GK (2017) Development of vanadium based hydrogen storage material: a review. Renew Sustain Rev 72:791–800. https://doi.org/10.1016/j.rser.2017.01.063
Xueping Z, Xiangsheng Y, Xinyue L, Runnan Yongsheng Z, Zhilao Z, Xushao H, Yan Z, Gang Z, Yiging P (2019) Hydrogen storage performance of HPSB hydrogen storage materials. Chem Phys Lett 734:1–7. https://doi.org/10.1016/j.cplett.2019.136697
Hunt JD, Byers E, Balogun A-L, Leal-Filho W, Colling AV, Nascimento A, Yoshihide Wada (2019) Using the jet stream for sustainable airship and balloon transportation of cargo and hydrogen. Energy Convers Manag 3:1000161–10001610. https://doi.org/10.1016/j.ecmx.2019.100016
Ley MB, Jepsen LH, See Y-Su, Cho YW, von Colbe JMB, Dornhein M, Rokni M, Jensen JO, Sloth M, Filinchu KY, Jørsen JE, Resenbacher F, Jesnen TR (2014) Complex hydrides for hydrogen storage: new Perspectives. Mat Today 17:122–127. https://doi.org/10.1016/j.mattod.2014.02.013
Yang J, Sudik A, Wolverton C, Siegelw DJ (2010) High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem Soc Rev 39:656–675. https://doi.org/10.1039/b802882f
Toyta Mirai https://en.wikipedia.org/wiki/Toyota_Mirai
Towards clean and future-oriented mobility (2019) https://www.alstom.com/our-solutions/rolling-stock/coradia-ilint-worlds-1st-hydrogen-powered-train
World′s first hydrogen train rolls out in Germany. https://www.dw.com/en/worlds-first-hydrogen-train-rolls-out-in-germany/a-45525062
Züttel A (2003) Materials for hydrogen storage. Mater Today 6:24–33. https://doi.org/10.1016/S1369-7021(03)00922-2
Crivello J-C, Denys RV, Dornheim M, Felderhoff M, Grant DM, Huot J, Rde Jensen T, Jongh Latroche PM, Walker GS, Webb CJ, Yartys VA (2016) Mg-based compounds for hydrogen and energy storage. Appl Phys 122:1–17. https://doi.org/10.1007/s00339-016-9601-1
Oliveira ACM, Pavão AC (2018) Theoretical study of hydrogen storage in metal hydrides. J Mol Model 24:1–8. https://doi.org/10.1007/s00894-018-3661-4
Li J, Furuta T, Goto H, Ohashi T, Fujiwara Y, Yip S (2003) Theoretical evaluation of hydrogen storage capacity in pure carbon nanostructures. J Chem Phys 119:2376–2385. https://doi.org/10.1063/1.1582831
Wang L, Yang RT (2008) New sorbents for hydrogen storage by hydrogen spillover: a review. Energy Environ Sci 1:268–279. https://doi.org/10.1039/B807957A
Tsivion E, Long JR, Head-Gordon M (2014) Hydrogen physisorption on metal-organic framework linkers and Metalated Linkers: a computational study of the factors that control binding strength. J Am Chem Soc 136:17827–17835. https://doi.org/10.1021/ja5101323
DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles, https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles
Chandrakunar KS, Ghosh SK (2008) Alkali-metal-induced enhancement of hydrogen adsorption in C60 fullerene: an ab initio study. Nano Lett 8:13–19. https://doi.org/10.1021/nl071456i
Chen Y, Wang J, Yuan L, Zhang M, Zhang C (2017) Sc-decorated porous graphene for high-capacity hydrogen storage: first-principles calculations. Materials 10:1–11. https://doi.org/10.3390/ma10080894
Senithurai S, Pandyan RK, Kumar SV, Saranya M, Mahendran M (2014) Al-decorated carbon nanotube as the molecular hydrogen storage medium. Int. J. Hydrog Energy 34:11990–11998. https://doi.org/10.1016/j.ijhydene.2014.05.184
Kundu A, Piccini GM, Sillar K, Sauer J (2016) Ab Initio prediction of adsorption isotherms for small molecules in metal–organic frameworks. J Am Chem Soc 138:14047–14056. https://doi.org/10.1021/jacs.6b08646
Maia RA, Oliveira FL, Nazarkovsky M, Esteves PM (2018) Crystal engineering of covalent organic frameworks based on hydrazine and hydroxy-1,3,5-triformylbenzenes. Cryst Growth Des 18:5682–5689. https://doi.org/10.1021/acs.cgd.8b01110
Bhatia SK, Myers AL (2006) Optimum conditions for adsorptive storage. Langmuir 22:1688–1700. https://doi.org/10.1021/la0523816
Arean CO, Bonelli B, Delgado MR, Garrone E (2009) Hydrogen storage via physisorption: the combined role of adsorption enthalpy and entropy. Turk J Chem 33:599–606. https://doi.org/10.3906/kim-0812-22
Shinde R, Tayade M (2014) Remarkable hydrogen storage on beryllium oxide cluster: first-principles calculations. J Phys Chem C 118:17200–17204. https://doi.org/10.1021/jp4109943
Ren L, Cheng L, Feng Y, Wang X (2012) Geometric and electronic structures of (BeO)N (N = 2–12, 16, 20, and 24): rings, double, and cages. J Chem Phys 137:014309
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski R, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009), Gaussian 09. Gaussian Inc, Wallingford, CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Adamo C, Barrone V (1999) Toward to reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170. https://doi.org/10.1063/1.478522
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2002) Climbing the density functional ladder: nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:1464011–1464014. https://doi.org/10.1103/PhysRevLett.91.146401
Zhao Y, Truhlar DG (2006) A new local density functional for main group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101. https://doi.org/10.1063/1.2370993
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. https://doi.org/10.1039/b810189b
Szabo A, Ostlund NS (1996) Modern quantum chemistry: introduction to advanced electronic structure theory. Dover, New York, p 320
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electronic correlation theories. Chem Phys Lett 157:479–483. https://doi.org/10.1016/S0009-2614(89)87395-6
Hehre WJ, Ditchfield R, Pople JAJ (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Baba M, Kowaka Y, Nagashima U, Ishimoto T, Goto H, Nakayama N (2011) Exact agreement of rotational constants between ultrahigh resolution spectroscopy and ab initio calculation. J Chem Phys 135:054305. https://doi.org/10.1063/1.3622766
Carvalho EFV, Lopez-Castillo A, Roberto-Neto O (2018) A comparative study of the structures and electronic properties of graphene fragments: a DFT and MP2 survey. Chem Phys Lett 691:291–297. https://doi.org/10.1016/j.cplett.2017.11.023
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Papajak E, Zheng J, Xu X, Leverentz HR, Truhlar DG (2011) Perspectives on basis sets beautiful: seasonal plantings of diffuse basis functions. J Chem Phys Theory Comput 7:3027–3033. https://doi.org/10.1021/ct200106a
Grimme S (2011) Density functional theory with London dispersion corrections. Adv Rev 1:211–227
Burns L, Vázquez-Mayagoitia A, Sumpter BG, Sherrill CD (2011) Density-functional approaches to noncovalent interactions: a comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functional. J Chem Phys 134:084107–08410725. https://doi.org/10.1063/1.3545971
Peverati R, Truhlar DG (2014) Quest for a universal density functional: the accuracy of density functionals across a broad spectrum of databases in chemistry and physics. Philos Trans R Soc A 372:1–52. https://doi.org/10.1098/rsta.2012.0476
Lichanot A, Chaillet M, Larrieu C, Dovesi R, Pisani C (1992) Ab initio Hartree-Fock study of solid beryllium oxide: structure and electronic properties. Chem Phys 164:383–394. https://doi.org/10.1016/0301-0104(92)87076-L
Perdew JP, Schmidt K (2001) Jacob’s ladder of density functional approximations for the exchange correlation energy. AIP Conf Proc 577:1–9. https://doi.org/10.1063/1.1390175
Lee TJ, Taylor PR (1989) A diagnostic for determining the quality of single-reference electron correlation methods. Int J Quant Chem 23:199–207. https://doi.org/10.1002/qua.560360824
Halkier A, Helgaker T, Jorgensen P, Klopper W, Koch H, Olsen A, Wilson AK (1998) Basis-set convergence in correlated calculations on Ne, N, and H2O. Chem Phys Lett 286:243–252. https://doi.org/10.1016/S0009-2614(98)00111-0
Huber KP, Herzberg G (1979) Constants of diatomic molecules. Van Nostrand Reinhold, New York
Karton A, Martin JML (2010) Performance of W4 theory for spectroscopic constants and electrical properties of small molecules. J Chem Phys 133:1441021–14410217. https://doi.org/10.1063/1.3489113
Murthy NS, Prakllad UD (1978) True potential energy curve and dissociation energy of BeO J Phys B: Atom. Mol Phys 11:825–829. https://doi.org/10.1088/0022-3700/11/5/017
Luo Y-R (2009) Bond Dissociation Energies http://staff.ustc.edu.cn/~luo971/2010-91-CRC-BDEs-Tables.pdf
Irizawa J, Iwata S (1992) Ab initio studies of the low-lying states of BeO. Theor Chim Acta 81:223–254. https://doi.org/10.1007/BF01118563
Pontes MAP, Machado FBC, Roberto-Neto O, Ferrão LFA (2020) Soot precursors in farnesane and n-dodecane decomposition: a computational approach. Fuel 268:117334. https://doi.org/10.1016/j.fuel.2020.117334
PVR Schleyer, Maerker C, Fransfeld A, Jiao H, van Eikema Hommes NJ (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318. https://doi.org/10.1021/ja960582d
Luo S, Zhao Y, Truhlar DG (2012) Improved CO adsorption energies, site preferences, and surface formation energies from a meta-generalized gradient approximation exchange–correlation functional M06-L. J Phys Chem Lett 3:2975–2979. https://doi.org/10.1021/jz301182a
Acknowledgements
We acknowledge the research support of the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) underprocess no. 01049/18, and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under Processes Nos. 480119/2012-0 and 422647/2016-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Festschrift in honor of Prof. Fernando R. Ornellas” Guest Edited by Adélia Justino Aguiar Aquino, Antonio Gustavo Sampaio de Oliveira Filho & Francisco Bolivar Correto Machado.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roberto-Neto, O., de Carvalho, E.F.V. A DFT and wave function theory study of hydrogen adsorption on small beryllium oxide clusters. Theor Chem Acc 139, 93 (2020). https://doi.org/10.1007/s00214-020-02605-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02605-z