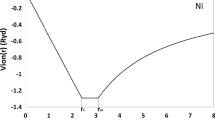
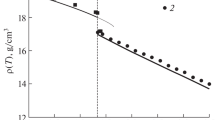
Abstract
In this study, Monte Carlo simulations were carried out to detect the metal–insulator transition in liquid mercury by changing the static properties at the microscopic scale. In the simulations pair, empirical, and many-body potentials govern metal–metal interactions in the canonical ensemble. The structural and thermodynamic changes over a wide temperature range from 773 to 1773 K are described in the first coordination shell and residual internal energy, respectively. The results reveal that during the simulated heating process, the properties undergo significant change at 1673 K, which is in connection with the metal-nonmetal transition in the liquid. These findings coincide with the experimental observations of this thermodynamic phenomenon. Notably, the free energy of association that renders the system to this thermodynamic state is estimated.
Graphical Abstract






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Spencer AJ (2000) Dental amalgam and mercury in dentistry. Aust Dent J 45:224–234. https://doi.org/10.1111/j.1834-7819.2000.tb00256.x
Iakovlev A, Bedrov D, Müller M (2015) Surface tension of liquid mercury: a comparison of density-dependent and density-independent force fields. Eur Phys J B 88:1. https://doi.org/10.1140/epjb/e2015-60594-2
Iakovlev A, Bedrov D, Müller M, (2016) Alkyl-based surfactants at a liquid mercury surface: computer simulation of structure, self-assembly, and phase behavior. J Phys Chem Lett 7:1546–1553. https://doi.org/10.1021/acs.jpclett.6b00494
Elfassy E, Mastai Y, Pontoni D, Deutsch M (2016) Liquid-mercury-supported Langmuir films of ionic liquids: isotherms, structure, and time evolution. Langmuir 32:3164–3173. https://doi.org/10.1021/acs.langmuir.6b00196
Sun X, Hwang JY, Xie S (2011) Density functional study of elemental mercury adsorption on surfactants. Fuel 90:1061–1068. https://doi.org/10.1016/j.fuel.2010.10.043
Galashev AY (2016) Modeling of forced desorption processes in a regenerable graphene sorbent for elemental mercury capture. J Phys Chem C 120:13263–13274. https://doi.org/10.1021/acs.jpcc.6b02826
Abbas T, Gonfa G, Lethesh KC, Mutalib MA, btAbai M, Cheun KY, Khan E (2016) Mercury capture from natural gas by carbon supported ionic liquids: synthesis, evaluation and molecular mechanism. Fuel 177:296–303. https://doi.org/10.1016/j.fuel.2016.03.032
Liu Y, Adewuyi YG (2016) A review on removal of elemental mercury from flue gas using advanced oxidation process: chemistry and process. Chem Eng Res Des 112:199–250. https://doi.org/10.1016/j.cherd.2016.06.024
Polishuk I, Nakonechny F, Brauner N (2016) Predicting phase behavior of metallic mercury in liquid and compressed gaseous hydrocarbons. Fuel 174:197–205. https://doi.org/10.1016/j.fuel.2016.02.002
Landau LD, Zeldovich YB (1943) On the relation between the liquid and the gaseous states of metals. Acta Physicochim USSR 18:194–196
Kozhevnikov VF, Arnold DI, Naurzakov SP (1995) Experimental study of phase transitions in mercury. Int J Thermophys 16:619–628. https://doi.org/10.1007/BF01438847
Hoshino K, Tanaka S, Shimojo F (2007) Dynamical structure of fluid mercury: molecular-dynamics simulations. J Non Cryst Solids 353:3389–3393. https://doi.org/10.1016/j.jnoncrysol.2007.05.089
Kresse G (1996) Ab initio molecular dynamics applied to the dynamics of liquid metals and to the metal-non-metal transition. J Non Cryst Solids 205:833–840. https://doi.org/10.1016/S0022-3093(96)00314-6
Calderín L, González LE, González DJ (2011) Static dynamic and electronic properties of expanded fluid mercury in the metal–nonmetal transition range An ab initio study. J Condens Matter Phys 23:375105. https://doi.org/10.1088/0953-8984/23/37/375105
Hong X (2007) Structural variation of expanded fluid mercury during M-NM transition: a reverse Monte Carlo study. J Non Cryst Solids 353:3399–3404. https://doi.org/10.1016/j.jnoncrysol.2007.05.091
Ghatee MH, Bahadori M (2004) Density-dependent equations of state for metal, nonmetal, and transition states for compressed mercury fluid. J Phys Chem B 108:4141–4146. https://doi.org/10.1021/jp035940v
Endo H (1984) Metal-nonmetal transitions in liquids under pressure. J Non Cryst Solids 61:1–2. https://doi.org/10.1016/0022-3093(84)90522-2
Kitamura H (2007) The role of attractive many-body interaction in the gas–liquid transition of mercury. J Condens Matter Phys 19:072102. https://doi.org/10.1088/0953-8984/19/7/072102
Nagel S, Redmer R, Röpke G (1996) Equation of state and structure factor of mercury. J Non Cryst Solids 205:247–250. https://doi.org/10.1016/S0022-3093(96)00232-3
Kobayashi K, Kajikawa H, Hiejima Y, Hoshino T, Yao M (2007) A precursor of liquid–liquid coexistence in the metal–nonmetal transition range of fluid mercury. J Non Cryst Solids 353:3362–3365. https://doi.org/10.1016/j.jnoncrysol.2007.05.084
Ayrinhac S, Gauthier M, Bove LE, Morand M, Le Marchand G, Bergame F, Philippe J, Decremps F (2014) Equation of state of liquid mercury to 520 K and 7 GPa from acoustic velocity measurements. J Chem Phys 140:244201. https://doi.org/10.1063/1.4882695
Inui M, Ishikawa D, Matsuda K, Tamura K, Baron AQ (2008) Experimental techniques of high-resolution inelastic X-ray scattering measurements for supercritical metallic fluids at high temperature and high pressure using synchrotron radiation at SPring-8. J Phys Condens Matter. https://doi.org/10.5488/CMP.11.1.83
Inui M, Matsuda K, Ishikawa D, Tamura K, Ohishi Y (2007) Medium-range fluctuations accompanying the metal-nonmetal transition in expanded fluid hg. Phys Rev Lett 98:185504. https://doi.org/10.1103/PhysRevLett.98.185504
Levin M, Schmutzler RW (1984) The specific heat of mercury at sub-and supercritical temperatures and pressures. J Non Cryst Solids 61:83–88. https://doi.org/10.1016/0022-3093(84)90534-9
Guell OA, Holcombe JA (1990) Analytical applications of Monte Carlo techniques. Anal Chem 62:529A-A542. https://doi.org/10.1021/ac00208a001
Karimi H, Ghatee MH, Davatolhagh S (2019) Modeling the annealing and thermal stability of silver dental amalgam. J Mol Model 25:1–8. https://doi.org/10.1007/s00894-019-4193-2
Toth G (2003) An iterative scheme to derive pair potentials from structure factors and its application to liquid mercury. J Chem Phys 118:3949–3955. https://doi.org/10.1063/1.1543142
Belashchenko DK (2006) Application of the embedded atom model to liquid metals: Liquid mercury. High Temp 44:675–686. https://doi.org/10.1007/s10740-006-0082-3
Belashchenko DK (2002) The simulation of liquid mercury by diffraction data and the inference of interparticle potential. High Temp 40:212–221. https://doi.org/10.1023/A:1015299006040
Raabe G, Sadus RJ (2003) Molecular simulation of the vapor–liquid coexistence of mercury. J Chem Phys 119:6691–6697. https://doi.org/10.1063/1.1605381
Belashchenko DK (2013) Application of the embedded atom model to liquid mercury. High Temp 51:40–48. https://doi.org/10.1134/S0018151X13010021
Belashchenko DK (2013) Erratum to: “Application of the embedded atom model to liquid mercury.” High Temp 51:878. https://doi.org/10.1134/S0018151X13070018
Bomont JM, Bretonnet JL (2006) An effective pair potential for thermodynamics and structural properties of liquid mercury. J Chem Phys 124:054504. https://doi.org/10.1063/1.2166384
Ghatee MH, Karimi H, Shekoohi K (2015) Structural, mechanical and thermodynamical properties of silver amalgam filler: a Monte Carlo simulation study. J Mol Liq 211:96–104. https://doi.org/10.1016/j.molliq.2015.06.062
Tao DP (2005) Prediction of the coordination numbers of liquid metals. Metall Mater Trans A 36:3494–3497. https://doi.org/10.1007/s11661-005-0023-5
Tamura K, Inui M, Matsuda K, Ishikawa D (2007) Structural instability and the metal–nonmetal transition in expanded fluid metals. J Non Cryst Solids 353:3348–3357. https://doi.org/10.1016/j.jnoncrysol.2007.05.124
Inui M, Ishikawa D, Matsuda K, Tamura K, Tsutsui S, Baron AQ (2005) Observation of fast sound in metal–nonmetal transition in liquid Hg. J Phys Chem Solids 66:2223–2229. https://doi.org/10.1016/j.jpcs.2005.09.021
Tamura K, Hosokawa S (1998) Structural studies of expanded fluid mercury up to the liquid-vapor critical region. Phys Rev B 58:9030. https://doi.org/10.1103/PhysRevB.58.9030
Franz JR (1986) Metal-nonmetal transition in expanded liquid mercury. Phys Rev lett 57:889. https://doi.org/10.1103/PhysRevLett.57.889
Timko J, De Castro A, Kuyucak S (2011) Ab initio calculation of the potential of mean force for dissociation of aqueous Ca–Cl. J Chem Phys 134:204510. https://doi.org/10.1063/1.3595261
Song W, Biswas R, Maroncelli M (2000) Intermolecular interactions and local density augmentation in supercritical solvation: a survey of simulation and experimental results. J Phys Chem A 104:6924–6939. https://doi.org/10.1021/jp000888d
Desgranges C, Delhommelle J (2014) Thermodynamics of phase coexistence and metal–nonmetal transition in mercury: assessment of effective potentials via expanded Wang-Landau simulations. J Phys Chem B 118:3175–3182. https://doi.org/10.1021/jp500577t
Acknowledgements
The author is indebted to the research council of the Shiraz University for the financial supports. Also, the author would like to express his sincere thanks to Prof. Belashchenko from the Moscow State Institute of Steel and Alloys, and Prof. U.K. Deiters from the University of Cologne for their discussions, and Dr. Jean-Marc Bomont from the University of Lorraine for supplied g (r) data.
Author information
Authors and Affiliations
Contributions
Hedayat Karimi: conceptualization, methodology, investigation, and writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karimi, H. Computer simulation and modeling the metal to insulating transition of liquid mercury via pair, empirical, and many-body potentials. J Mol Model 28, 377 (2022). https://doi.org/10.1007/s00894-022-05372-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05372-9