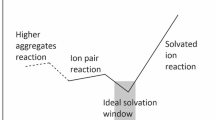
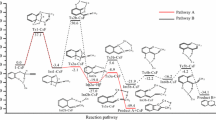
Abstract
The reactivity of the fluoride ion towards alkyl halides is highly dependent on the solvating environment. In polar aprotic solvents with large counter-ions is highly reactive and produces substantial E2 product, whereas in polar protic solvents leads to slow kinetics and high selectivity for SN2 reactions. The use of a more complex environment with stoichiometric addition of tert-butanol to acetonitrile solvent is able to module the reactivity and selectivity of tetrabutylammonium fluoride (TBAF). In the present work, we have performed a detailed theoretical analysis of this complex reaction system by density functional theory, continuum solvation model, and including explicit tert-butanol molecules. A kinetic model based on the free energy profile was also used to predict the reactivity and selectivity. The results indicated that the TBAF(tert-butanol) complex plays the key role to increase the SN2 selectivity, whereas higher aggregates are not relevant. The E2 product is formed exclusively via free TBAF, because the solvating tert-butanol in the TBAF(tert-butanol) complex inhibits the E2 pathway. Our analysis suggests that diols or tetraols could produce an improved selectivity.
Graphical abstract






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Reichardt C (2022) Solvation effects in organic chemistry: a short historical overview. J Org Chem 87:1616–1629
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry. Fourth edn. WILEY-VCH, Weinheim, Germany
Herbert JM (2021) Dielectric continuum methods for quantum chemistry. Wiley Interdiscip Rev Comput Mol Sci 11:e1519
Voityuk AA, Vyboishchikov SF (2020) Fast and accurate calculation of hydration energies of molecules and ions. Phys Chem Chem Phys 22:14591–14598
Klamt A (2018) The COSMO and COSMO-RS solvation models. Wiley Interdiscip Rev Comput Mol Sci 8:e1338
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Lipparini F, Mennucci B (2016) Perspective: polarizable continuum models for quantum-mechanical descriptions. J Chem Phys 144:160901
Struebing H, Ganase Z, Karamertzanis PG, Siougkrou E, Haycock P, Piccione PM, Armstrong A, Galindo A, Adjiman CS (2013) Computer-aided molecular design of solvents for accelerated reaction kinetics. Nat Chem 5:952–957
Struebing H, Obermeier S, Siougkrou E, Adjiman CS, Galindo A (2017) A QM-CAMD approach to solvent design for optimal reaction rates. Chem Eng Sci 159:69–83
Dalessandro EV, Pliego JR Jr (2018) Solvent selection for chemical reactions: automated computational screening of solvents using the SMD model. Quim Nova 41:628–633
Dalessandro EV, Pliego JR Jr (2018) Fast screening of solvents for simultaneous extraction of furfural, 5-hydroxymethylfurfural and levulinic acid from aqueous solution using SMD solvation free energies. J Brazil Chem Soc 29:430–434
Blumenthal LC, Jens CM, Ulbrich J, Schwering F, Langrehr V, Turek T, Kunz U, Leonhard K, Palkovits R (2016) Systematic identification of solvents optimal for the extraction of 5-hydroxymethylfurfural from aqueous reactive solutions. ACS Sustain Chem Eng 4:228–235
Das M, Gogoi AR, Sunoj RB (2022) Molecular insights on solvent effects in organic reactions as obtained through computational chemistry tools. J Org Chem 87:1630–1640
Norjmaa G, Ujaque G, Lledós A (2021) Beyond continuum solvent models in computational homogeneous catalysis. Top Catal 65:118–140
Sunoj RB, Anand M (2012) Microsolvated transition state models for improved insight into chemical properties and reaction mechanisms. Phys Chem Chem Phys 14:12715–12736
Rufino VC, Pliego JR (2021) Single-ion solvation free energy: a new cluster-continuum approach based on the cluster expansion method. Phys Chem Chem Phys 23:26902–26910
Pliego JR Jr (2017) Cluster expansion of the solvation free energy difference: systematic improvements in the solvation of single ions. J Chem Phys 147:034104
Pliego JR Jr, Riveros JM (2020) Hybrid discrete-continuum solvation methods. Wiley Interdiscip Rev Comput Mol Sci 10:e1440
Anslyn EV, Dougherty DA (2006) Modern physical organic chemistry. University Science Books Sausalito, CA
Pliego JR (2021) The role of intermolecular forces in ionic reactions: the solvent effect, ion-pairing, aggregates and structured environment. Org Biomol Chem 19:1900–1914
Liotta CL, Harris HP (1974) Chemistry of naked anions. I. Reactions of the 18-crown-6 complex of potassium fluoride with organic substrates in aprotic organic solvents. J Am Chem Soc 96:2250–2252
Silva SL, Valle MS, Pliego JR (2020) Nucleophilic fluorination with KF catalyzed by 18-crown-6 and bulky diols: a theoretical and experimental study. J Org Chem 85:15457–15465
Silva SL, Valle MS, Pliego JR (2020) Micro-solvation and counter ion effects on ionic reactions: activation of potassium fluoride with 18-crown-6 and tert-butanol in aprotic solvents. J Mol Liq 319:114211
Jadhav VH, Jeong HJ, Choi W, Kim DW (2015) Crown ether metal complex fluoride salt as a facile and low hygroscopic fluoride source for nucleophilic fluorination. Chem Eng J 270:36–40
Schwesinger R, Link R, Wenzl P, Kossek S (2006) Anhydrous phosphazenium fluorides as sources for extremely reactive fluoride ions in solution. Chem Eur J 12:438–445
Pliego JR (2018) Potassium fluoride activation for the nucleophilic fluorination reaction using 18-crown-6, [2.2.2]-cryptand, pentaethylene glycol and comparison with the new hydro-crown scaffold: a theoretical analysis. Org Biomol Chem 16:3127–3137
Han HJ, Lee S-S, Kang SM, Kim Y, Park C, Yoo S, Kim CH, Oh Y-H, Lee S, Kim DW (2020) The effects of structural modifications of bis-tert-alcohol-functionalized crown-calix[4]arenes as nucleophilic fluorination promotors and relations with computational predictions. Eur J Org Chem 2020:728–735
Kang SM, Kim CH, Lee KC, Kim DW (2019) Bis-triethylene glycolic crown-5-calix[4]arene: a promoter of nucleophilic fluorination using potassium fluoride. Org Lett 21:3062–3066
Jadhav VH, Choi W, Lee S-S, Lee S, Kim DW (2016) Bis-tert-alcohol-functionalized crown-6-calix[4]arene: an organic promoter for nucleophilic fluorination. Chem Eur J 22:4515–4520
Pliego JR (2018) Mechanism of nucleophilic fluorination promoted by bis-tert-alcohol-functionalized crown-6-calix[4]arene. Int J Quantum Chem 118:e25648
Dalessandro EV, Pliego JR (2020) Theoretical design of new macrocycles for nucleophilic fluorination with KF: thiourea-crown-ether is predicted to overcome [2.2.2]-cryptand. Mol Syst Des Eng 5:1513–1523
Jadhav VH, Jang SH, Jeong H-J, Lim ST, Sohn M-H, Kim J-Y, Lee S, Lee JW, Song CE, Kim DW (2012) Oligoethylene glycols as highly efficient mutifunctional promoters for nucleophilic-substitution reactions. Chem Eur J 18:3918–3924
Lee JW, Yan H, Jang HB, Kim HK, Park SW, Lee S, Chi DY, Song CE (2009) Bis-terminal hydroxy polyethers as all-purpose, multifunctional organic promoters: a mechanistic investigation and applications. Angew Chem, Int Ed 48:7683–7686
Kim J-Y, Kim DW, Song CE, Chi DY, Lee S (2013) Nucleophilic substitution reactions promoted by oligoethylene glycols: a mechanistic study of ion-pair SN2 processes facilitated by Lewis base. J Phys Org Chem 26:9–14
Kim DW, Jeong H-J, Lim ST, Sohn M-H (2010) Facile nucleophilic fluorination of primary alkyl halides using tetrabutylammonium fluoride in a tert-alcohol medium. Tetrahedron Lett 51:432–434
Kim DW, Jeong H-J, Lim ST, Sohn M-H (2008) Tetrabutylammonium tetra(tert-butyl alcohol)-coordinated fluoride as a facile fluoride source. Angew Chem, Int Ed 47:8404–8406
Engle KM, Pfeifer L, Pidgeon GW, Giuffredi GT, Thompson AL, Paton RS, Brown JM, Gouverneur V (2015) Coordination diversity in hydrogen-bonded homoleptic fluoride-alcohol complexes modulates reactivity. Chem Sci 6:5293–5302
Cox DP, Terpinski J, Lawrynowicz W (1984) Anhydrous tetrabutylammonium fluoride - a mild but highly efficient source of nucleophilic fluoride-ion. J Org Chem 49:3216–3219
Landini D, Maia A, Rampoldi A (1989) Dramatic effect of the specific solvation on the reactivity of quaternary ammonium fluorides and poly(hydrogen fluorides), (Hf)-normal.F-, in media of low polarity. J Org Chem 54:328–332
Albanese D, Landini D, Penso M (1998) Hydrated tetrabutylammonium fluoride as a powerful nucleophilic fluorinating agent. J Org Chem 63:9587–9589
Maldonado AM, Basdogan Y, Berryman JT, Rempe SB, Keith JA (2020) First-principles modeling of chemistry in mixed solvents: where to go from here? J Chem Phys 152:130902
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Zheng JJ, Xu XF, Truhlar DG (2011) Minimally augmented Karlsruhe basis sets. Theor Chem Acc 128:295–305
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Neese F, Wennmohs F, Becker U, Riplinger C (2020) The ORCA quantum chemistry program package. J Chem Phys 152:224108
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci 2:73–78
McQuarrie DA (2000) Statistical mechanics. University Science Books, Sausalito, CA
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol Phys 115:2315–2372
Barca GMJ, Bertoni C, Carrington L, Datta D, Silva ND, Deustua JE, Fedorov DG, Gour JR, Gunina AO, Guidez E, Harville T, Irle S, Ivanic J, Kowalski K, Leang SS, Li H, Li W, Lutz JJ, Magoulas I, Mato J, Mironov V, Nakata H, Pham BQ, Piecuch P, Poole D, Pruitt SR, Rendell AP, Roskop LB, Ruedenberg K, Sattasathuchana T, Schmidt MW, Shen J, Slipchenko L, Sosonkina M, Sundriyal V, Tiwari A, Vallejo JLG, Westheimer B, Włoch M, Xu P, Zahariev F, Gordon MS (2020) Recent developments in the general atomic and molecular electronic structure system. J Chem Phys 152:154102
Xu X, Zhang Q, Muller RP, Goddard WA III (2005) An extended hybrid density functional (X3LYP) with improved descriptions of nonbond interactions and thermodynamic properties of molecular systems. J Chem Phys 122:014105–014114
Dalessandro EV, Pliego JR (2020) Reactivity and stability of ion pairs, dimers and tetramers versus solvent polarity: SNAr fluorination of 2-bromobenzonitrile with tetramethylammonium fluoride. Theor Chem Acc 139:27
Sun H, DiMagno SG (2005) Anhydrous tetrabutylammonium fluoride. J Am Chem Soc 127:2050–2051
Funding
The authors thank the agencies CNPq, FAPEMIG, and CAPES for support.
Author information
Authors and Affiliations
Contributions
Josefredo R. Pliego Jr. contributed to the study conception and design. Material preparation and data collection were performed by Fernando M. Lisboa. Analysis was performed by Fernando M. Lisboa and Josefredo R. Pliego Jr. The first draft of the manuscript was written by Josefredo R. Pliego Jr. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection: XXI-Brazilian Symposium of Theoretical Chemistry (SBQT2021).
Supplementary information
The coordinates of the optimized structures are available.
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lisboa, F.M., Pliego, J.R. SN2 versus E2 reactions in a complex microsolvated environment: theoretical analysis of the equilibrium and activation steps of a nucleophilic fluorination. J Mol Model 28, 159 (2022). https://doi.org/10.1007/s00894-022-05160-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05160-5