Abstract
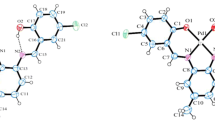
The present work reports the theoretical investigation of Co(II), Ni(II), and Zn(II) complexes containing Schiff bases (used as ligands) derived from the reaction of 2-hydroxy-1-naphthaldehyde with N-(2-aminoethyl) pyrazoles. The spectral analyses were carried out using infrared, Raman, and UV-Vis spectroscopy. Vibrational analyses were performed in order to investigate the mechanisms involving metal-ligand and intra-ligand vibrations and indicated the possibility of charge transfer related to the transitions n\(\rightarrow \pi\)* and \(\pi \rightarrow \pi\)*. Structure optimizations and normal coordinate force field calculations were performed via the density functional theory (DFT) method at the HSE06/6-311G(d,p)/LanL2DZ level. A thorough analysis was also conducted regarding the nonlinear optical (NLO) properties and the natural bond orbital (NBO) of the complexes. The results show that these complexes have prospective application as materials for NLO. Furthermore, the NBO analysis confirms the coordination between the lone pair (LP) electrons of the donor atoms (O and N) and the metal acceptors. Finally, studies were conducted regarding the electronic properties of the complexes; among the properties investigated included the frontier molecular orbitals (FMO) and the molecular electrostatic potential (MEP), allowing to determine the energy gap and charge distribution.
Graphical abstract








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Elshaarawy RFM, Refaee AA, El-Sawi EA (2016) Pharmacological performance of novel poly-(ionic liquid)-grafted chitosan-n-salicylidene Schiff bases and their complexes. Carbohydr Polym 146:376–387. https://doi.org/10.1016/j.carbpol.2016.03.017
Abu-Dief AM, Mohamed IMA (2015) A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-suef Univ J Basic Appl Sci 4(2):119–133. https://doi.org/10.1016/j.bjbas.2015.05.004
Sakthivel A, Thalavaipandian A, Raman N, Thangagiri B (2017) Synthesis, characterization and antifungal activity of transition metal (II) complexes ofSchiff base derived from p-amino acetanilide and salicylaldehyde. Int J Curr Sci Res 3(5):1253–1260
Hassan MA, Omer AM, Abbas E, Baset WMA, Tamer TM (2018) Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci Rep 8(1):1–14. https://doi.org/10.1038/s41598-018-29650-w
More MS, Joshi PG, Mishra YK, Khanna PK (2019) Metal complexes driven from Schiff bases and semicarbazones forbiomedical and allied applications: a review. Mater Today Chem 14:100195. https://doi.org/10.1016/j.mtchem.2019.100195
Chen Y, Li P, Su S, Chen M, He J, Liu L, He M, Wang H, Xue W (2019) Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1, 2, 4-triazole Schiff base. RSC Adv 9(40):23045–23052. https://doi.org/10.1039/C9RA05139B
Barbosa HFG, Attjioui M, Ferreira APG, Dockal ER, El Gueddari NE, Moerschbacher BM, Cavalheiro ÉTG (2017) Synthesis, characterization and biological activities of biopolymeric Schiff bases prepared with chitosan and salicylaldehydes and theirPd(II) and Pt(II) complexes. Molecules 22(11):1987. https://doi.org/10.3390/molecules22111987
de Araújo EL, Barbosa HFG, Dockal ER, Cavalheiro ÉTG (2017) Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int J Biol Macromol 95:168–176. https://doi.org/10.1016/j.ijbiomac.2016
de Fátima Â, de Paula Pereira C, Olímpio CRSDG, de Freitas Oliveira BG, Franco LL, da Silva PHC (2018) Schiff bases and their metal complexes as urease inhibitors–a brief review. J Adv Res 13:113–126. https://doi.org/10.1016/j.jare.2018.03.007
Salehi M, Faghani F, Kubicki M, Bayat M (2018) New complexes of Ni(II) and Cu(II) with tridentate ONO Schiff base ligand: synthesis, crystal structures, electrochemical and theoretical investigation. J Iran Chem Soc 15(10):2229–2240. https://doi.org/10.1007/s13738-018-1412-1
Li Z, Yan H, Chang G, Hong M, Dou J, Niu M (2016) Cu(II), Ni(II) complexes derived from chiral Schiff-base ligands: Synthesis, characterization, cytotoxicity, protein and DNA-binding properties. J Photochem Photobiol B Biol 163:403–412. https://doi.org/10.1016/j.jphotobiol.2016.09.005
Adak P, Mondal A, Chattopadhyay SK (2020) Manganese (II) complex of an oxygen-nitrogen donor Schiff base ligand showing efficient catechol oxidase activity: synthesis, spectroscopic and kinetic study. New J Chem 44(9):3748–3754. https://doi.org/10.1039/C9NJ04591K
Angeluşiu MV, Almăjan GL, Ilieş DC, Roşu T, Negoiu M (2008) Cu(II) complexes with nitrogen-oxygen donor ligands: synthesis and biological activity. Chem. Bull. “POLITEHNICA’’ Univ.(Timisoara) 53:78–82
Winpenny REP (2001) High-nuclearity paramagnetic 3d-metal complexes with oxygen-and nitrogen-donor ligands. Adv Inorg Chem 52:1–11. https://doi.org/10.1016/S0898-8838(05)52001-4
Patel DD, Patel KR (2020) Ni(II) and Zn(II) Schiff base complexes: Synthesis, characterization and study of thermodynamic parameters and activation energy. Mater Today: Proc 32:392–396. https://doi.org/10.1016/j.matpr.2020.02.079
Beyramabadi SA, Saadat-Far M, Faraji-Shovey A, Javan-Khoshkholgh M, Morsali A (2020) Synthesis, experimental and computational characterizations of a new quinoline derived Schiff base and its Mn(II), Ni(II) and Cu(II) complexes. J Mol Struct 1208:127898. https://doi.org/10.1016/j.molstruc.2020.127898
Joshi R, Kumari A, Singh K, Mishra H, Pokharia S (2019) Synthesis, structural characterization, electronic structure calculation, molecular docking study and biological activity of triorganotin (IV) complexes of Schiff base (E)-4-amino-3-(2-(2-hydroxybenzylidene) hydrazinyl)-1H-1, 2, 4-triazole-5 (4H)-thione. J Mol Struct 1197:519–534. https://doi.org/10.1016/j.molstruc.2019.07.066
Malekshah RE, Shakeri F, Khaleghian A, Salehi M (2020) Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni(II) and Zn(II) complexes and biological evaluation as pro-drug. Int J Biol Macromol 152:846–861. https://doi.org/10.1016/j.ijbiomac.2020.02.245
Turkan F, Cetin A, Taslimi P, Karaman M, Gulçin I (2019) Synthesis, biological evaluation and molecular docking of novel pyrazole derivatives as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorg Chem 86:420–427. https://doi.org/10.1016/j.bioorg.2019.02.013
Milovanović V, Petrović ZD, Novaković S, Bogdanović GA, Simijonović D, Petrović VP (2019) Structural characterization of benzoyl-1H-pyrazole derivatives obtained in lemon juice medium: Experimental and theoretical approach. J Mol Struct 1195:85–94. https://doi.org/10.1016/j.molstruc.2019.05.095
Sadjadi S, Heravi MM, Daraie M (2017) A novel hybrid catalytic system based on immobilization of phosphomolybdic acid on ionic liquid decorated cyclodextrin-nanosponges: Efficient catalyst for the green synthesis of benzochromeno-pyrazole through cascade reaction: Triply green. J Mol Liquids 231:98–105. https://doi.org/10.1016/j.molliq.2017.01.072
Mayoral MJ, Ovejero P, Criado R, Lagunas MC, Pintado-Alba A, Torres MR, Cano M (2011) Diphosphines and pyrazole/pyrazolate-type ligands as building blocks in luminescent Au(I) complexes. J Organometal Chem 696(15–16):2789–2796. https://doi.org/10.1016/j.jorganchem.2011.04.022
Iskeleli NO, Alpaslan YB, Direkel Ş, Ertürk AG, Süleymanoğlu N, Ustabaş R (2015) The new Schiff base 4-[(4-Hydroxy-3-fluoro-5-methoxy-benzylidene) amino]-1, 5-dimethyl-2-phenyl-1, 2-dihydro-pyrazol-3-one: Experimental, DFT calculational studies and in vitro antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc 139:356–366. https://doi.org/10.1016/j.saa.2014.12.071
Layek S, Agrahari B, Pathak DD et al (2017) Synthesis and characterization of a new Pd(II)-Schiff base complex [Pd(APD) 2]: An efficient and recyclable catalyst for Heck-Mizoroki and Suzuki-Miyaura reactions. J Organomet Chem 846:105–112. https://doi.org/10.1016/j.jorganchem.2017.05.049
Gama S, Mendes F, Marques F, Santos IC, Carvalho MF, Correia I, Pessoa JC, Santos I, Paulo A (2011) Copper(II) complexes with tridentate pyrazole-based ligands: synthesis, characterization, DNA cleavage activity and cytotoxicity. J Inorg Biochem 105(5):637–644. https://doi.org/10.1016/j.jinorgbio.2011.01.013
Feng C, Guo J-J, Sun L-N, Zhao H (2018) Pyrazole Schiff bases cross-linked supramolecules: structural elucidation and antibacterial activity. J Iranian Che Soc 15(12):2871–2876. https://doi.org/10.1007/s13738-018-1473-1
Moreno-Fuquen R, Cuenú F, Torres JE, De la Vega G, Galarza E, Abonia R, Kennedy AR (2017) Presence of π ...π and CH ...π interactions in the new Schiff base 2-{(E)-[(3-tert-butyl-1-phenyl-1H-pyrazol-5-yl) imino] methyl} phenol: experimental and DFT computational studies. J Mol Struct 150:366–373. https://doi.org/10.1016/j.molstruc.2017.08.093
Moreira JM, Campos GF, Pinto LMC, Martins GR, Tirloni B, Schwalm CS, Carvalho CT (2022) Copper (II) complexes with novel schiff-based ligands: synthesis, crystal structure, thermal (TGA-DSC/FT-IR), spectroscopic (FT-IR, UV-Vis) and theoretical studies. J Therm Anal Calorim 147:4087–4098. https://doi.org/10.1007/s10973-021-10803-5
Heyd J, Scuseria GE (2004) Efficient hybrid density functional calculations in solids: Assessment of the Heyd-Scuseria-Ernzerhof screened Coulomb hybrid functional. J Chem Phys 121(3):1187–1192. https://doi.org/10.1063/1.1760074
Yahiaoui S, Moliterni A, Corriero N, Cuocci C, Toubal K, Chouaih A, Djafri A, Hamzaoui F (2019) 2-thioxo-3n-(2-methoxyphenyl)- 5 [4’-methyl-3’ n-(2’-methoxyphenyl) thiazol-2’(3’ h)-ylidene] thiazolidin-4-one: Synthesis, characterization, X-ray single crystal structure investigation and quantum chemical calculations. J Mol Struct 1177:186–192. https://doi.org/10.1016/j.molstruc.2018.09.052
Wadt WR, Hay PJ (1985) Ab initio effective core potentials formolecular calculations. potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–305. https://doi.org/10.1063/1.448800
Alagawani S, Vasilyev V, Wang F (2021) Benchmarking the performance of DFT functionals for absorption and fluorescence spectra of EGFR inhibitor AG-1478 using TD-DFT. arXiv:2112.03441
Sabzyan H, Keshavarz E, Noorisafa Z (2015) Evaluation of the B3LYP and HSE06 density functionals in the calculation of spectroscopic properties of the hcl\(^{2+}\) dication. J Iranian Chem Soc 12(4):581–586. https://doi.org/10.1007/s13738-014-0515-6
Tamer Ö, Avcı D, Atalay Y (2017) A novel Cu(ii) complex of picolinate and 1, 10-phenanthroline: Preparation, crystal structure determination, spectroscopic characterization and nonlinear optical studies. J Inorg Organomet Polym Mater 27(3):700–713. https://doi.org/10.1007/s10904-017-0513-0
Avcı D, Bahçeli S, Tamer Ö, Atalay Y (2015) Comparative study of DFT/B3LYP, B3PW91, and HSEH1PBE methods applied to molecular structures and spectroscopic and electronic properties of flufenpyr and amipizone. Canadian J Chem 93(10):1147–1156. https://doi.org/10.1139/cjc-2015-0176
Linh TPT, Hieu NN, Phuc HV, Nguyen CQ, Vinh PT, Thai NQ, Hieu NV (2021) First-principles insights onto structural, electronic and optical properties of janus monolayers CrXO (X = S, Se, Te). RSC Advances 11(63):39672–39679. https://doi.org/10.1039/D1RA07876C
Mathew T, Sujith CP, Mathew V (2021) Electronic and optical properties of Quasi-1D barium zinc chalcogenides Ba\(_{2}\)ZnX\(_3\) (X = S, Se, Te): A DFT approach. Solid State Sci 113:106456. https://doi.org/10.1016/j.solidstatesciences.2020.106456
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB (2016) Fox, DJ Gaussian16 Revision C.01. Gaussian Inc. Wallingford CT. https://gaussian.com
Dennington R, Keith TA, Millam JM (2019) GaussView Version 6. Semichem Inc. Shawnee Mission KS. https://gaussian.com
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926. https://doi.org/10.1021/cr00088a005
Jamróz MH (2013) Vibrational Energy Distribution Analysis (VEDA): Scopes and limitations. Spectrochim Acta A Mol Biomol Spectrosc 114:220–230. https://doi.org/10.1016/j.saa.2013.05.096
Irving HMNH, Williams RJP (1953) 637 the stability of transition-metal complexes. J Chem Soc 3192–3210. https://doi.org/10.1039/JR9530003192
Atkins P, Overton T (2010) Shriver and Atkins’ Inorganic Chemistry, 5th edn. Oxford University Press, New York
Tsuneda T, Song J-W, Suzuki S, Hirao K (2010) On Koopmans’ theorem in density functional theory. J Chem Phys 133(17):174101. https://doi.org/10.1063/1.3491272
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102(11):1995–2001. https://doi.org/10.1021/jp9716997
Arroio A, Honório KM, da Silva ABF (2010) Quantum chemical properties used in structure-activity relationship studies. Química Nova 33(3):694–699. https://doi.org/10.1590/S0100-40422010000300037
Thanikaivelan P, Subramanian V, Rao JR, Nair BU (2000) Application of quantum chemical descriptor in quantitative structure activity and structure property relationship. Chem Phys Lett 323(1–2):59–70. https://doi.org/10.1016/S0009-2614(00)00488-7
Reed AE, Weinhold F (1983) Natural bond orbital analysis of near-Hartree-Fock water dimer. J Chem Phys 78(6):4066–4073. https://doi.org/10.1063/1.445134
Carpenter JE (1987) Extension of Lewis structure concepts to open-shell and excited-state molecular species. PhD thesis, University of Wisconsin, Madison-WI. https://search.library.wisc.edu/catalog/999585226502121
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins’’ natural bond orbital procedure. J Mol Struct Theo Chem 169:41–62. https://doi.org/10.1016/0166-1280(88)80248-3
Shoba D, Periandi S, Boomadevi S, Ramalingam S, Fereyduni E (2014) FT-IR, FT-Raman, UV, NMR spectra, molecular structure, ESP, NBO and HOMO-LUMO investigation of 2-methylpyridine 1-oxide: A combined experimental and DFT study. Spectrochim Acta A Mol Biomol Spectrosc 118:438–447. https://doi.org/10.1016/j.saa.2013.09.023
Singh P, Islam SS, Ahmad H, Prabaharan A (2018) Spectroscopic investigation (FT-IR, FT-Raman), HOMO-LUMO, NBO, and molecular docking analysis of N-ethyl-N-nitrosourea, a potential anticancer agent. J Mol Struct 1154:39–50. https://doi.org/10.1016/j.molstruc.2017.10.012
Savithiri S, Rajarajan G, Thanikachalam V et al (2016) Molecular structure, vibrational spectral assignments (FT-IR and FT-Raman), UV-Vis, NMR, NBO, HOMO-LUMO and NLO properties of 3t-pentyl-2r, 6c-diphenylpiperidin-4-one picrate based on DFT calculations. J Mol Struct 1105:225–237. https://doi.org/10.1016/j.molstruc.2015.10.063
Prasad GK, Prashanth S, Srivastava S, Rao GN, Babu DR (2017) Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1, 10-phenanthroline-2, 9-di (carboxaldehyde phenylhydrazone) and its transition metal complexes. Open Chem 15(1):283–292. https://doi.org/10.1515/chem-2017-0036
Kanis DR, Ratner MA, Marks TJ (1994) Design and construction of molecular assemblies with large second-order optical nonlinearities. Quantum chemical aspects. Chem Rev 94(1):195–242. https://doi.org/10.1021/cr00025a007
Sajan D, Joe H, Jayakumar VS, Zaleski J (2006) Structural and electronic contributions to hyperpolarizability in methyl p-hydroxy benzoate. J Mol Struct 785(1–3):43–53. https://doi.org/10.1016/j.molstruc.2005.09.041
Demircioğlu Z, Kaştaş ÇA, Büyükgüngör O (2015) The spectroscopic (FT-IR, UV-vis), Fukui function, NLO, NBO, NPA and tautomerism effect analysis of (E)-2-[(2-hydroxy-6-methoxybenzylidene) amino] benzonitrile. Spectrochim Acta A Mol Biomol Spectrosc 139:539–548. https://doi.org/10.1016/j.saa.2014.11.078
Sylvestre S, Sebastian S, Edwin S, Amalanathan M, Ayyapan S, Jayavarthanan T, Oudayakumar K, Solomon S (2014) Vibrational spectra (FT-IR and FT-Raman), molecular structure, natural bond orbital, and TD-DFT analysis of L-Asparagine monohydrate by density functional theory approach. Spectrochim Acta A Mol Biomol Spectrosc 133:190–200. https://doi.org/10.1016/j.saa.2014.05.040
Muthu S, Porchelvi EE (2013) FTIR, FT-RAMAN, NMR, spectra, normal co-ordinate analysis, NBO, NLO and DFT calculation of N, N-diethyl-4-methylpiperazine-1-carboxamide molecule. Spectrochim Acta A Mol Biomol Spectrosc115:275–286. https://doi.org/10.1016/j.saa.2013.06.011
Avcı D, Altürk S, Sönmez F, Tamer Ö, Başoğlu A, Atalay Y, Kurt BZ, Dege N (2020) Novel metal complexes containing 6-methylpyridine-2-carboxylic acid as potent \({\alpha }\)-glucosidase inhibitor: synthesis, crystal structures, DFT calculations, and molecular docking. Mol Divers, 1–19. https://doi.org/10.1007/s11030-020-10037-x
Bredas JL, Adant C, Tackx P, Persoons A, Pierce BM (1994) Third-order nonlinear optical response in organic materials: theoretical and experimental aspects. Chem Rev 94(1):243–278. https://doi.org/10.1021/cr00025a008
Sebastian S, Sundaraganesan N, Karthikeiyan B, Srinivasan V (2011) Quantum mechanical study of the structure and spectroscopic (FT-IR, FT-Raman, 13C, 1H and UV), first order hyperpolarizabilities, NBO and TD-DFT analysis of the 4-methyl-2-cyanobiphenyl. Spectrochim Acta A Mol Biomol Spectrosc 78(2):590–600. https://doi.org/10.1016/j.saa.2010.11.028
Altürk S, Avcı D, Başoğlu A, Tamer Ö, Atalay Y, Dege N (2018) Copper (II) complex with 6-methylpyridine-2-carboxyclic acid: experimental and computational study on the XRD, FT-IR and UV-Vis spectra, refractive index, band gap and NLO parameters. Spectrochim Acta A Mol Biomol Spectrosc 190:220–230. https://doi.org/10.1016/j.saa.2017.09.041
Avcı D (2011) Second and third-order nonlinear optical properties and molecular parameters of azo chromophores: semiempirical analysis. Spectrochim Acta A Mol Biomol Spectrosc 82(1):37–43. https://doi.org/10.1016/j.saa.2011.06.037
Avcı D (2010) The consistency analysis of different semiempirical calculations on second-and third-order nonlinear optical properties of donor-acceptor chromophores containing a-cyan. Spectrochim Acta A Mol Biomol Spectrosc 77(3):665–672. https://doi.org/10.1016/j.saa.2010.07.007
El Kouari Y, Migalska-Zalas A, Arof AK, Sahraoui B (2015) Computations of absorption spectra and nonlinear optical properties of molecules based on anthocyanidin structure. Optic Quant Electron 47(5):1091–1099. https://doi.org/10.1007/s11082-014-9965-4
Wu K, Snijders JG, Lin C (2002) Reinvestigation of hydrogen bond effects on the polarizability and hyperpolarizability of urea molecular clusters. J Phys Chem B 106(35):8954–8958. https://doi.org/10.1021/jp014181i
Adant C, Dupuis M, Bredas JL (1995) Ab initio study of the nonlinear optical properties of urea: electron correlation and dispersion effects. Int J Quant Chem 56(S29):497–507. https://doi.org/10.1002/qua.560560853
Ferrero M, Civalleri B, Rérat M, Orlando R, Dovesi R (2009) The calculation of the static first and second susceptibilities of crystalline urea: A comparison of Hartree-Fock and density functional theory results obtained with the periodic coupled perturbed Hartree-Fock/Kohn-Sham scheme. J Chem Phy 131(21):214704. https://doi.org/10.1063/1.3267861
Abbas H, Shkir M, AlFaify S (2019) Density functional study of spectroscopy, electronic structure, linear and nonlinear optical properties of l-proline lithium chloride and l-proline lithium bromide monohydrate: For laser applications. Arab J Chem 12(8):2336–2346. https://doi.org/10.1016/j.arabjc.2015.02.011
Altürk S, Avcı D, Tamer Ö, Atalay Y, Şahin O (2016) A cobalt (II) complex with 6-methylpicolinate: Synthesis, characterization, second-and third-order nonlinear optical properties, and DFT calculations. J Phys Chem Solids 98:71–80. https://doi.org/10.1016/j.jpcs.2016.06.008
Nalwa HS, Miyata S (1996) Nonlinear optics of organic molecules and polymers, 1st edn. CRC Press, London. https://doi.org/10.1201/9780138745493
Funding
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grant 434138/2018-5) for the financial grant provided in support of this research. The study was partly financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Contributions
Gabriel Rodrigues Martins: methodology, formal analysis and investigation, writing–original draft preparation; Cristiane Storck Schwalm: conceptualization, writing–review and editing; Cláudio Teodoro de Carvalho: conceptualization, formal analysis and investigation, writing–review and editing; Leandro Moreira de Campos Pinto: conceptualization, methodology, formal analysis and investigation, writing–original draft preparation, funding acquisition, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martins, G.R., Schwalm, C.S., Carvalho, C.T.d. et al. Co(II), Ni(II), and Zn(II) complexes based on new hybrid imine-pyrazole ligands: structural, spectroscopic, and electronic properties. J Mol Model 28, 162 (2022). https://doi.org/10.1007/s00894-022-05109-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05109-8