Abstract
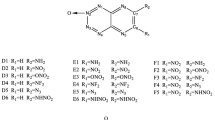
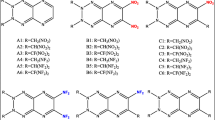
A series of bridged tetrazine derivatives (BDDT) were designed by using different bridges to connect two molecules of 1,2,4, 5-tetrazine oxides and then combining different substituents. At the same time, we used DFT-wB97/6–31 + G** method to regularly predict the HOMO–LUMO, heats of formation (HOF), detonation properties, thermal stability, and thermodynamic property orbitals of BDDT compounds. By studying the comprehensive relationship between different substituents and bridging and performance, it is shown that -N(NO2)2 and -C(NO2)3 are not only excellent groups to improve the heat of formation and detonation properties, but also can cause the compound to have a superior oxygen balance. And that the incorporation of the -N = N- and -NH-N = N- is helpful to enhance their thermal stabilities and HOF. -CH2-CH2- and -CH2-NH- are good for improving the HOMO–LUMO energy gaps. Performances with positive HOF (1170–1590 kJ mol−1), remarkable density (1.88–1.93 g cm−3), outstanding detonation properties (D = 9.15–9.80 km s−1, P = 38.24–44.40 GPa), and acceptable impact sensitivity lead C5, D8, E5, E7, F5, and F7 to be the potential candidates of HEDMs.












Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The application or custom code are available from the corresponding author upon reasonable request.
References
Jia YF, Ma Q, Zhang ZQ, Geng WJ, Huang JL, Yang W, Fan GJ, Wang SM (2020) Energetic 1H-imidazo[4,5-d]pyridazine-2,4,7-triamine: a novel nitrogen-rich fused heterocyclic cation with high density. Cryst Growth Des 20:3406–3412
Wu JT, Xu J, Li W, Li HB (2020) Coplanar fused heterocycle-based energetic materials. Propellants Explos Pyrotech 45:536–545
Tang YX, He CL, Imler GH, Parrish DA, Shreeve JM (2019) Aminonitro groups surrounding a fused pyrazolotriazine ring: a superior thermally stable and insensitive energetic material. Acs Appl Energy Mater 2:2263–2267
Pan Y, Zhu WH, Xiao HM (2013) Theoretical studies on the structures, heats of formation, energetic properties and pyrolysis mechanisms of nitrogen-rich difurazano[3,4-b:3′,4′-e]piperazine derivatives and their analogues. Struct Chem 24:1071–1087
Solomos MA, Bertke JA, Swift JA (2018) One step synthesis of a fused four-ring heterocycle. New J Chem 42:7125–7129
Steele BA, Oleynik II (2017) Novel potassium polynitrides at high pressures. J Phys Chem A 121:8955–8961
Zhang J, Shreeve JM (2014) 3,3′-dinitroamino-4,4′-azoxyfurazan and its derivatives: an assembly of diverse N-O building blocks for high-performance energetic materials. J Am Chem Soc 136:4437–4444
Kumar D, Imler GH, Parrish DA, Shreeve JM (2017) A highly stable and insensitive fused triazolotriazine explosive (TTX). Chem Eur J 23:1743–1747
Zhang J, Shreeve JM (2014) Thermally stable 3, 6dinitropyrazolo [4, 3c] pyrazole based energetic materials. Chem Asian J 9:2953–2960
Yin P, He C, Shreeve JM (2016) Fused heterocyclebased energetic salts: alliance of pyrazole and 1, 2, 3triazole. J Mater Chem A 4:1514–1519
Klenov MS, Guskov AA, Anikin OV, Churakov AM, Strelenko YA, Fedyanin IV, Lyssenko KA, Tartakovsky VA (2016) Synthesis of tetrazinotetrazine 1,3,6,8tetraoxide (TTTO). Angew Chem Int Ed 55:11472–11475
Tang Y, Kumar D, Shreeve JM (2017) Balancing excellent performance and high thermal stability in a dinitropyrazole fused 1, 2, 3, 4tetrazine. J Am Chem Soc 139:13684–13687
Kaihoh T, Itoh T, Yamaguchi K, Ohsawa A (1988) First synthesis of a 1, 2, 3, 4tetrazine. J Chem Soc Chem Commun 1:1608–1609
Thottempudi V, Yin P, Zhang J, Parrish DA, JnM S (2014) 1,2,3Triazolo [4,5, e] furazano [3,4, b] pyrazine 6oxide—a fused heterocycle with a roving hydrogen forms a new class of insensitive energetic materials. Chem Eur J 20:542–548
Piercey DG, Chavez DE, Scott BL, Imler GH, Parrish DA (2016) An energetic triazolo 1,2,4 triazine and its N⁃oxide. Angew Chem Int Ed 55:15315–15318
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann E, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Iiskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Chen BJW, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.01. Gaussian Inc., Pittsburgh
Byrd EF, Rice BM (2006) J Phys Chem A 110:1005
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Murray JS, Politzer P (1994) Quantitative treatment of solute/solvent interactions, Theoretical and Computational Chemistry. Elsevier Scientific, Amsterdam
Politzer P, Murray JS, Brinck T, Lane P (1994) Immunoanalysis of agrochemicals, ACS Symp Ser 586. American Chemical Society, Washington, DC
Atkins PW (1982) Physical chemistry. Oxford University Press, Oxford
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C-H–N–O explosives. J Chem Phys 48:23–35
Kamlet MJ, Ablard JE (1968) Chemistry of detonations. II. Buffered equilibria. J Chem Phys 48:36–42
Kamlet MJ, Dickinson C (1968) Chemistry of detonations. III. Evaluation of the simplified calculational method for Chapman-Jouguet detonation pressures on the basis of available experimental information. J Chem Phys 48:43–50
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem 106:1770–1783
Ryu J, Bok T, Joo SH, Yoo S, Song G, Kim SH, Choi S, Jeong HY, Kim MG, Kang SJ, Wang C, Kwak SK, Park S (2021) Electrochemical scissoring of disordered silicon-carbon composites for high-performance lithium storage. Energy Storage Mater 36:139–146
Zhong M, DaZou XuYB, Jin LJ, Pan Y (2021) Effect of kaolinites modified with Zr and transition metals on the pyrolysis behaviors of low-rank coal and its model compound. J Energy Inst 95:41–51
Tang YX, Imer GH, Parrish DA, Shreeve JM (2019) Energetic and fluorescent azole-fused 4-amino-1,2,3-triazine-3-N-oxides. Acs Appl Energy Mater 2:8871–8877
Suvitha A, Periandy S, Boomadevi S, Govindarajan M (2014) Vibrational frequency analysis, FT-IR, FT-Raman, ab initio, HF and DFT studies, NBO, HOMO-LUMO and electronic structure calculations on pycolinaldehyde oxime. Spectrochim Acta A 117:216–224
Kosar B, Albayrak C (2011) Spectroscopic investigations. and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino)methyl]phenol. Spectrochim Acta A 78:160–167
Simpson RL, Urtiew PA, Ornellas DL, Moody GL, Scribner KJ, Hoffman DM (1997) Propellants Explos Pyrotech 22:249
Politzer P, Murray JS (2011) Some perspectives on estimating detonation properties of C, H, N, O compounds. Cent Eur J Energy Mater 8:209–220
Jeong K (2018) New theoretically predicted RDX- and -HMX-based high-energy-density molecules. Int J Quantum Chem 118:e25528
He P, Zhang JG, Wang K, Yin X, Jin X, Zhang TL (2015) Extensive theoretical studies on two new members of the FOX-7 family: 5-(dinitromethylene)-1,4-dinitramino-tetrazole and 1,1 ′-dinitro-4,4 ′-diamino-5,5 ′-bitetrazole as energetic compounds. Phys Chem Chem Phys 17:5840–5848
Pan Y, Zhu WH, Xiao HM (2012) Design and selection of nitrogen-rich bridged di-1,3,5-triazine derivatives with high energy and reduced sensitivity. J Mol Model 18:3125–3138
Zhu WH, Zhang CC, Wei T, Xiao HM (2011) Computational study of energetic nitrogen-rich derivatives of 1,1 ′- and 5,5 ′-bridged ditetrazoles. J Comput Chem 32:2298–2312
Zhai LJ, Bi FQ, Zhang JL, Zhang JR, Li XZ, Wang BZ, Chen SP (2020) 3,4-Bis(3-tetrazolylfuroxan-4-yl)furoxan: a linear C-C bonded pentaheterocyclic energetic material with high heat of formation and superior performance. ACS Omega 5:11115–11122
Pu KY, Wang LY, Liu J, Zhong K (2020) Theoretical design of bis-azole derivatives for energetic compounds. RSC Adv 10:13185–13195
Borisov YA, Makarenkov AV, Kiselev SS, Kononova EG, Ponomaryov AB, Budnik MI, Ol’shevskaya VA (2020) Prediction of energetic properties of carboranyl tetrazoles based on DFT study. Mater Chem Phys 240:122209
Lin H, Zhu Q, Huang C, Yang DD, Lou N, Zhu SG, Li HZ (2019) Dinitromethyl, fluorodinitromethyl derivatives of RDX and HMX as high energy density materials: a computational study. Struct Chem 30:2401–2408
Srivastava P, Singh HJ (2010) Computational studies on nitro-azaboriridinea high-energetic material. J Energy Mater 28:202–218
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propellants Explosive Pyrotech 41:414–425
Ghule VD (2012) Computational studies on the triazole-based high energy materials. Comput Theor Chem 992:92–96
Schmalz T, Seitz WA, Klein DJ, Hite G (1988) Elemental carbon gages. J Am Chem Soc 110:1113–1127
Zhang J, Xiao H (2002) Computational studies on the infrared vibrational spectra, thermodynamic properties, detonation properties, and pyrolysis mechanism of octanitrocubane. J Chem Phys 116:10674–10683
Qiu L, Xiao H, Gong X, Ju X, Zhu W (2006) Theoretical studies on the structures, thermodynamic properties, detonation properties, and pyrolysis mechanisms of spiro nitramines. J Phys Chem A 110:3797–3807
Lewczuk R, Ksiazek M, Ganczyk-Specjalska K, Cieslak K (2020) Structure and thermal properties of 2,2′-azobis(1H-imidazole-4,5-dicarbonitrile)-a promising starting material for a novel group of energetic compounds. Molecules 25:314–324
Ma Q, Jiang T, Zhang XY, Fan GJ, Wang J, Huang JL (2015) Theoretical investigations on 4,4 ′,5,5 ′-tetranitro-2,2 ′-1H,1 ′ H-2,2 ′-biimidazole derivatives as potential nitrogen-rich high energy materials. J Phys Org Chem 28:31–39
Funding
This work was supported by Opening Project of State Key Laboratory of Explosion Science and Technology (Beijing Institute of Technology) (NO. KFJJ20-03 M), Doctoral Fundation of SWUST (NO. 17zx7128), and Major Special Projects of the Equipment Development Department of the Central Military Commission of China (NO. 14021001040305–5).
Author information
Authors and Affiliations
Contributions
Lian Zeng: the first author, conceive and write the manuscript; Junyan Li, Chen Qiao, and Yuhe Jiang: data collection; Jinting Wu, Hongbo Li: corresponding author; Jianguo Zhang: methods to guide.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, L., Li, J., Qiao, C. et al. Combination multi-nitrogen with high heat of formation: theoretical studies on the performance of bridged 1,2,4,5-tetrazine derivatives. J Mol Model 28, 3 (2022). https://doi.org/10.1007/s00894-021-04999-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04999-4