Abstract
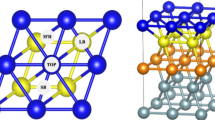
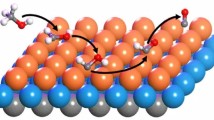
The mechanism of formic acid decomposition on the Pd(111) surface has been investigated by several theoretical methods in previous studies, including PBE and PW91. These results indicated that the mechanism is different from different methods, and even by using the same method (i.e., PBE), the mechanism is also different. In this study, we have revisited the formic acid decomposition on Pd(111) surface by using another density functional RPBE and by including van der Waals interaction which is neglected in the previous studies. Our results showed that the formic acid is decomposed via O–H bond cleavage to form bi-HCOO*, and the most favorable pathway is HCOOH* → bi-HCOO* + H* → CO2* + 2H*. The energy barrier is 0.55 eV at the rate-determining step. This conclusion is consistent with one of the PBE study. This demonstrated that computational methods have a great influence on the reaction mechanism, and care should be taken in selecting the appropriate computational methods.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
VASP software package.
References
Aricò A, Bruce P, Scrosati B, Tarascon JM, Schalkwijk WV (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377. https://doi.org/10.1038/nmat1368
Johnson TC, Morris DJ, Wills M (2010) Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem Soc Rev 39:81–88. https://doi.org/10.1039/B904495G
Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski A, Barnard T (2002) Direct formic acid fuel cells. J Power Sources 111:83–89. https://doi.org/10.1016/S0378-7753(02)00271-9
Wang X, Hu JM, Hsing IM (2004) Electrochemical investigation of formic acid electro-oxidation and its crossover through a Nafion membrane. J Eletroanal Chem 562:73–80. https://doi.org/10.1016/j.jelechem.2003.08.010
Zhu Y, Ha SY, Masel RI (2004) High power density direct formic acid fuel cells. J Power Sources 130:8–14. https://doi.org/10.1016/j.jpowsour.2003.11.051
Yu XW, Pickup PG (2008) Recent advances in direct formic acid fuel cell (DFAFC). J Power Sources 182:124–132. https://doi.org/10.1016/j.jpowsour.2008.03.075
Ozensoy E, Min BK, Santra AK, Goodman DW (2004) CO dissociation at elevated pressures on supported Pd nanoclusters. J Phys Chem B 108:4351–4357. https://doi.org/10.1021/jp030928o
Davis JL, Barteau MA (1991) Reactions of carboxylic acids on the Pd(111)-(2×2)O surface: multiple roles of surface oxygen atoms. Surf Sci 256:50–66. https://doi.org/10.1016/0039-6028(91)91199-8
Patra S, Viswanath B, Barai K, Ravishankar N, Munichandraiah N (2010) High-surface step density on dendritic Pd leads to exceptional catalytic activity for formic acid oxidation. ACS Appl Mater Interfaces 2:2965–2969. https://doi.org/10.1021/am100647u
Mori K, Dojo M, Yamashita H (2013) Pd and Pd-Ag nanoparticles within a macroreticular basic resin: an efficient catalyst for hydrogen production from formic acid decomposition. ACS Catal 3:1114–1119. https://doi.org/10.1021/cs400148n
Lv Q, Meng Q, Liu W, Sun N, Jiang K, Ma L et al (2018) Pd-PdO interface as active site for HCOOH selective dehydrogenation at ambient condition. J Phys Chem C 122:2081–2088. https://doi.org/10.1021/acs.jpcc.7b08105
Karakurt B, Kocak Y, Ozensoy E (2019) Enhancement of formic acid dehydrogenation selectivity of Pd(111) single crystal model catalyst surface via Brønsted bases. J Phys Chem C 123:28777–28788. https://doi.org/10.1021/acs.jpcc.9b08707
Luo Q, Feng G, Beller M, Jiao H (2012) Formic acid dehydrogenation on Ni(111) and comparison with Pd(111) and Pt(111). J Phys Chem C 116:4149–4156. https://doi.org/10.1021/jp209998r
Zhang R, Liu H, Wang B, Ling L (2012) Insights into the preference of CO2 formation from HCOOH decomposition on Pd surface: a theoretical study. J Phys Chem C 116:22266–22280. https://doi.org/10.1021/jp211900z
Wang Y, Qi Y, Zhang D, Liu C (2014) New insight into the decomposition mechanism of formic acid on Pd(111): competing formation of CO2 and CO. J Phys Chem C 118:2067–2076. https://doi.org/10.1021/jp410742p
Herron JA, Scaranto J, Ferrin P, Li S, Mavrikakis M (2014) Trends in formic acid decomposition on model transition metal surfaces: a density functional theory study. ACS Catal 4:4434–4445. https://doi.org/10.1021/cs500737p
He F, Li K, Xie G, Wang Y, Jiao M, Tang H, Wu ZJ (2016) Understanding the enhanced catalytic activity of Cu1@Pd3(111) in formic acid dissociation, a theoretical perspective. J Power Sources 316:8–16. https://doi.org/10.1016/j.jpowsour.2016.03.062
Chen BWJ, Mavirkakis M (2020) Formic acid: a hydrogen-bonding cocatalyst for formate decomposition. ACS Catal 10:10812–10825. https://doi.org/10.1021/acscatal.0c02902
Wang JY, Zhang HX, Jiang K, Cai WB (2011) From HCOOH to CO at Pd electrodes: a surface-enhanced infrared spectroscopy study. J Am Chem Soc 133:14876–14879. https://doi.org/10.1021/ja205747j
Bulushev DA, Beloshapkin S, Ross JRH (2010) Hydrogen from formic acid decomposition over Pd and Au catalysts. Catal Today 154:7–12. https://doi.org/10.1016/j.cattod.2010.03.050
He N, Li ZH (2016) Palladium-atom catalyzed formic acid decomposition and the switch of reaction mechanism with temperature. Phys Chem Chem Phys 18:10005–10017. https://doi.org/10.1039/C6CP00186F
Yang L, Li G, Chang J, Ge J, Liu C, Vladdimir F, Wang G, Jin Z, XingW, (2020) Sea urchin-like Aucore@Pdshell electrocatalysts with high FAOR performance: Coefficient of lattice strain and electrochemical surface area. Appl Catal B 260:118200. https://doi.org/10.1016/j.apcatb.2019.118200
Tedsree K, Li T, Jones S, Chan CWA, Yu KMK, Bagot PAJ et al (2011) Hydrogen production from formic acid decomposition at room temperature using an Ag-Pd core-shell nanocatalyst. Nat Nanotechnol 6:302–307. https://doi.org/10.1038/nnano.2011.42
Yang M, Wang B, Li Z, Ling L, Zhang R (2020) HCOOH dissociation over the core-shell M@Pd bimetallic catalysts: probe into the effect of the core metal type on the catalytic performance. Appl Surf Sci 506:114938. https://doi.org/10.1016/j.apsusc.2019.144938
Cho J, Lee S, Yoon SP, Han J, Nam SW, Lee KY, Ham HC (2017) Role of heteronuclear interactions in selective H2 formation from HCOOH decomposition on bimetallic Pd/M (M = Late Transition FCC Metal) catalysts. ACS Catal 7:2553–2562. https://doi.org/10.1021/acscatal.6b02825
Jiang K, Xu K, Zou S, Cai WB (2014) B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid-formate solutions. J Am Chem Soc 136:4861–4864. https://doi.org/10.1021/ja5008917
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249. https://doi.org/10.1103/PhysRevB.45.13244
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865. https://doi.org/10.1103/PhysRevLett.77.3865
Li S, Rangarajan S, Scaranto J, Mavrikakis M (2021) On the structure sensitive of and CO coverage effects on formic acid decomposition on Pd surfaces. Surf Sci 709:121846. https://doi.org/10.1016/j.susc.2021.121846
Scaranto J, Mavrikakis M (2016) Density functional theory studies of HCOOH decomposition on Pd(111). Surf Sci 650:111–120. https://doi.org/10.1016/j.susc.2015.11.020
Hammer B, Hansen LB, Nørskov JK (1999) Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys Rev B 59:7413–7420. https://doi.org/10.1103/PhysRevB.59.7413
Yoo JS, Abild-Pedersen F, Nørskov JK, Studt F (2014) Theoretical analysis of transition-metal catalysts for formic acid decomposition. ACS Catal 4:1226–1233. https://doi.org/10.1021/cs400664z
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp Mater Sci 6:15–50. https://doi.org/10.1016/0927-0256(96)00008-0
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558. https://doi.org/10.1103/PhysRevB.47.558
Kresse G, Hafner J (1994) Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys Rev B 49:14251. https://doi.org/10.1103/PhysRevB.49.14251
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169. https://doi.org/10.1103/PhysRevB.54.11169
Blöchl PE, Kästner J, Först CJ (1994) Projector augmented-wave method. Phys Rev B 50:17953. https://doi.org/10.1103/PhysRevB.50.17953
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192. https://doi.org/10.1103/PhysRevB.13.5188
Methfessel M, Paxton AT (1989) High-precision sampling for Brillouin-zone integration in metals. Phys Rev B 40:3616. https://doi.org/10.1103/PhysRevB.40.3616
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Henkelman G, Uberuaga BP, Jónsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113:9901–9904. https://doi.org/10.1063/1.1329672
Lide DR, Frederikse EHIR (2002) CRC handbook of chemistry and physics, 83rd edn. New York, CRC Press
Hocking WH (1976) The other rotamer of formic acid, cis-HCOOH. Z. Naturforsch. 31a:1113–1121
Zhou SD, Qian C, Chen XZ (2011) Comparative theoretical study of adsorption and dehydrogenation of formic acid, hydrazine and isopropanol on Pd(111) surface. Catal Lett 141:726–734. https://doi.org/10.1007/s10562-011-0553-y
Sautet P, Rose MK, Dunphy JC, Behler S, Salmeron M (2000) Adsorption and energetics of isolated CO molecules on Pd(111). Surf Sci 453:25–31. https://doi.org/10.1016/S0039-6028(00)00243-0
Lininger CN, Gauthier JA, Li WL, Rossomme E, Welborn VV, Lin Z, Head-Gordon T, Head-Gordon M, Bell AT (2021) Challenges for density functional theory: calculation of CO adsorption on electrocatalyticlly relevant metals. Phys Chem Chem Phys 23:9394–9406. https://doi.org/10.1039/d0cp03821k
Abild-Pedersen F, Andersson MP (2007) CO adsorption energies on metals with correction for high coordination sites - a density functional study. Surf Sci 601:1747–1753. https://doi.org/10.1016/j.susc.2007.01.052
Felter TE, Sowa EC (1989) Location of hydrogen adsorbed on palladium (111) studied by low-energy electron diffraction. Phys Rev B 40:891. https://doi.org/10.1103/PhysRevB.40.891
Shustorovich E (1990) The bond-order conservation approach to chemisorption and heterogeneous catalysis: applications and implication. Adv Catal 37:101–163. https://doi.org/10.1016/S0360-0564(08)60364-8
Grimme S, Antony J, Ehrlich S, Krieg S (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Kozuch S, Shaik S (2011) How to conceptualize catalytic cycle? The energetic span model. Acc Chem Res 2:101–110. https://doi.org/10.1021/ar1000956
Acknowledgements
The computational time is supported by the high-performance computing center of Jilin University, computing center of Jilin Province, and computing center of Changchun Institute of Applied Chemistry.
Funding
This work is supported by the National Natural Science Foundation of China (21733004, 21673220) and the National Key Research and Development Program of China (2016YFA0602900).
Author information
Authors and Affiliations
Contributions
Ni Wang made the calculations, wrote the paper, and participated in the interpretation of the data. Kai Li and Ying Wang analyzed the data and revised the paper. Zhijian Wu proposed the project, participated in the structure design, mechanism analysis, and revision of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
The concept of the rate-determining steps and rate-determining states are discussed and the corresponding calculations based on the rate-determining states are listed in Figure S1. Table S1 is the calculated adsorption energies for the most stable adsorbed species on formic acid dissociation by RPBE with D2 and D3 method, respectively. (DOCX 43 KB)
Rights and permissions
About this article
Cite this article
Wang, N., Li, K., Wang, Y. et al. Density functional study on formic acid decomposition on Pd(111) surface: a revisit and comparison with other density functional methods. J Mol Model 27, 285 (2021). https://doi.org/10.1007/s00894-021-04903-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04903-0