Abstract
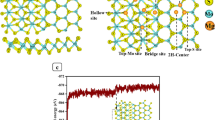
The adsorption and diffusion behaviors of magnesium (Mg) on monolayer Mo2C have been investigated by the first principles method based on density functional theory (DFT). The structural stability and theoretical capacity of monolayer Mo2C as anodes for magnesium-ion batteries (MIBs) have also been investigated. The results show that Mg prefer to occupy the H and TC sites with the adsorption energies of − 1.439 and − 1.430, respectively, followed by B and TMo sites on Mo2C monolayer. The Mg prefers to diffuse along the H-TC-H path, furthermore, the other two possible paths (along H-B-H and H-TMo-H) also possess quite low energy barrier with the value of about 0.039 eV. The present results demonstrate that the adsorption energy per Mg atom and the volume expansion change mildly. The volume expansions change slightly from 0.7 to 7.08% with the variety of x, ranging from 0.167 to 2.0. The theoretical gravimetric capacity reaches to 469.791 mAhg−1 with relatively small deformation and expansion as x = 2.0. The results mentioned above suggest that Mo2C monolayer is one of the promising candidates for anode material of MIBs.



Similar content being viewed by others
References
Zou F, Chen YM, Liu K, Yu Z, Liang W, Bhaway SM, Gao M, Zhu Y (2016) Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 10:377–386. https://doi.org/10.1021/acsnano.5b05041
Baek SH, Park JS, Jeong YM, Kim JH (2016) Facile synthesis of Ag-coated silicon nanowires as anode materials for high-performance rechargeable lithium battery. J Alloys Compd 660:387–391. https://doi.org/10.1016/j.jallcom.2015.11.131
Balogun MS, Yang L, Qiu W, Liu P, Tong Y (2016) A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon 98:162–178. https://doi.org/10.1016/j.carbon.2015.09.091
Zhao C, Wang X, Kong J, Ang JM, Lee PS, LiuZ LX (2016) Self-assembly-induced alternately stacked single-layer MoS2 and N-doped graphene: a novel van der Waals heterostructure for lithium-ion batteries. ACS Appl Mater Interfaces 8:2372–2379. https://doi.org/10.1021/acsami.5b11492
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657. https://doi.org/10.1038/451652a
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935. https://doi.org/10.1126/science.1212741
Tashiro Y, Taniguchi K, Miyasaka H (2016) Copper selenide as a new cathode material based on displacement reaction for rechargeable magnesium batteries. Electrochim Acta 210:655–661. https://doi.org/10.1016/j.electacta.2016.05.202
Matsui M (2011) Study on electrochemically deposited Mg metal. J Power Sources 196:7048–7055. https://doi.org/10.1016/j.jpowsour.2010.11.141
Li W, Yang Y, Zhang G, Zhang YW (2015) Ultrafast and directional diffusion of lithium in phosphorene for high-performance lithium-ion battery. Nano Lett 15:1691–1697. https://doi.org/10.1021/nl504336h
Wang Z, Su Q, Deng H, He W, Lin J, Fu YQ (2014) Modelling and simulation of electron-rich effect on Li diffusion in group IVA elements (Si, Ge and Sn) for Li ion batteries. J Mater Chem A 2:13976–13982. https://doi.org/10.1039/C4TA01614A
Zhang H, Cao D, Bai X (2019) High rate performance of aqueous magnesium-ion batteries based on the δ-MnO2@carbon molecular sieves composite as the cathode and nanowire VO2 as the anode. J Power Sources 444:227299. https://doi.org/10.1016/j.jpowsour.2019.227299
Yang S, Jia F, Wang Z, Zhu Y, Hu K, Ouyang YP, Wang R, Ma X, Cao C (2019) Microwave-assisted synthesis of CuSe nano-particles as a high-performance cathode for rechargeable magnesium batteries. Electrochim Acta 324:134864. https://doi.org/10.1016/j.electacta.2019.134864
Kravchyk KV, Widmer R, Erni R, Dubey RJC, Krumeich F, Kovalenko MV, Bodnarchuk MI (2019) Copper sulfide nanoparticles as high-performance cathode materials for Mg-ion batteries. Sci Rep 9:7988. https://doi.org/10.1038/s41598-019-43639-z
Ishibashi C, Mizutani Y, Ishida N, Kitamura N, Idemoto Y (2019) Crystal and electronic structures of MgCo2-xMnxO4 as cathode material for magnesium secondary batteries using first-principles calculations and quantum beam measurements. Bull Chem Soc Jpn 92:1950–1959. https://doi.org/10.1246/bcsj.20190207
Ni DX, Shi J, Xiong W, Zhong SY, Xu B, Ouyang CY (2019) The effect of protons on the Mg2+ migration in an α-V2O5 cathode for magnesium batteries: a first-principles investigation. Phys Chem Chem Phys 21:7406–7411. https://doi.org/10.1039/C9CP00528E
Vessally E, Alkorta I, Ahmadi S, Mohammadi R, Hosseinian A (2019) A DFT study on nanocones, nanotubes (4, 0), nanosheets and fullerene C, 60, as anodes in Mg-ion batteries. RSC Adv 9:853–862. https://doi.org/10.1039/C8RA06031B
Chockla AM, Harris JT, Akhavan VA, Bogart TD, Holmberg VC, Steinhagen C, Mullins CB, Stevenson KJ, Korgel BA (2011) Silicon nanowire fabric as a lithium ion battery electrode material. J Am Chem Soc 133:20914–20921. https://doi.org/10.1021/ja208232h
Hong W, Ge P, Jiang Y, Yang L, Tian Y, Zou G, Cao X, Hou H, Ji X (2019) Yolk-shell-structured bismuth@N-doped carbon anode for lithium-ion battery with high volumetric capacity. ACS Appl Mater Interfaces 11:10829–10840. https://doi.org/10.1021/acsami.8b20477
Chan KT, Neaton JB, Cohen ML (2008) First-principles study of metal adatom adsorption on graphene. Phys Rev B 77:235430. https://doi.org/10.1103/physrevb.77.235430
Zhao X, Hayner CM, Kung MC, Kung HH (2011) Flexible holey graphene paper electrodes with enhanced rate capability for energy storage applications. ACS Nano 5:8739–8749. https://doi.org/10.1021/nn202710s
Shao X, Wang K, Pang R, Shi XQ (2015) Lithium intercalation in graphene/MoS2 composites: first-principles insights. J Phys Chem C 119:10334–10344. https://doi.org/10.1021/acs.jpcc.5b06441
Wang Z, Chen Q, Wang J (2015) Electronic structure of twisted bilayers of graphene/MoS2 and MoS2/MoS2. J Phys Chem C 119:4752–4758. https://doi.org/10.1021/jp507751p
Cai Y, Zhang G, Zhang YW (2015) Electronic properties of phosphorene/graphene and phosphorene/hexagonal boron nitride heterostructures. J Phys Chem C 119:13929–13936. https://doi.org/10.1021/acs.jpcc.5b02634
Hu J, Xu B, Yang SA, Guan S, Ouyang C, Yao Y (2015) 2D electrides as promising anode materials for Na-ion batteries from first-principles study. ACS Appl Mater Interfaces 7:24016–24022. https://doi.org/10.1021/acsami.5b06847
Çakır D, Sevik C, Gülseren O, Peeters FM (2016) Mo2C as a high capacity anode material: a first-principles study. J Mater Chem A 4:6029–6035. https://doi.org/10.1039/C6TA01918H
Sun Q, Dai Y, Ma Y, Jing T, Wei W, Huang B (2016) Ab initio prediction and characterization of Mo2C monolayer as anodes for lithium-ion and sodium-ion batteries. J Phys Chem Lett 7:937–943. https://doi.org/10.1021/acs.jpclett.6b00171
Jin W, Wang Z, Fu YQ (2016) Monolayer black phosphorus as potential anode materials for Mg-ion batteries. J Mater Sci 51:7355–7360. https://doi.org/10.1007/s10853-016-0023-4
Han X, Cheng L, Jie S, Sendek A (2018) Density functional theory calculations for evaluation of phosphorene as a potential anode material for magnesium batteries. RSC Adv 8:7196–7204. https://doi.org/10.1039/C7RA12400G
Jing Y, Zhou Z, Cabrera CR, Chen Z (2013) Metallic VS2 monolayer: a promising 2D anode material for lithium ion batteries. J Phys Chem C 117:25409–25413. https://doi.org/10.1021/jp410969u
Yang E, Ji H, Jung Y (2015) Two-dimensional transition metal dichalcogenide monolayers as promising sodium ion battery anodes. J Phys Chem C 119:26374–26380. https://doi.org/10.1021/acs.jpcc.5b09935
Vakili-Nezhaada GR, Gujarathi AM, Rawahi NA, Mahnaz M (2019) Performance of WS2 monolayers as a new family of anode materials for metal-ion (Mg, Al and Ca) batteries. Mater Chem Phys 230:114–121. https://doi.org/10.1016/j.matchemphys.2019.02.086
Mortazavi B, Dianat A, Rahaman O, Cuniberti G, Rabczuk T (2016) Borophene as an anode material for Ca, Mg, Na or Li ion storage: a first-principle study. J Power Sources 329:456–461. https://doi.org/10.1016/j.jpowsour.2016.08.109
Xu C, Wang L, Liu Z, Chen L, Guo J, Kang N, Ma XL, Cheng HM, Ren W (2015) Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat Mater 14:1135–1141. https://doi.org/10.1038/nmat4374
Soler JM, Artacho E, Gale JD, García A, Junquera J, Ordejón P, Sánchez-Portal D (2002) The SIESTA method for ab initio order-N materials simulation. J Phys Condens Matter 14:2745–2779. https://doi.org/10.1088/0953-8984/14/11/302
White JA, Bird DM (1994) Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys Rev B 50:4954–4957. https://doi.org/10.1103/PhysRevB.50.4954
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/physrevlett.77.3865
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane-wave calculations. Phys Rev B Condens Matter 43:1993–2006. https://doi.org/10.1103/PhysRevB.43.1993
Drumm DW, Budi A, Per MC, Russo SP, Hollenberg LCL (2013) Ab initio calculation of valley splitting in monolayer δ-doped phosphorus in silicon. Nanoscale Res Lett 8:111. https://doi.org/10.1186/1556-276X-8-111
Hammouri M, Jha SK, Vasiliev I (2015) First-principles study of graphene and carbon nanotubes functionalized with benzyne. J Phys Chem C 119:18719. https://doi.org/10.1021/acs.jpcc.5b04065
Shi W, Wang Z, Li Z, Fu YQ (2016) Electric field enhanced adsorption and diffusion of adatoms in MoS2 monolayer. Mater Chem Phys 183:392–397. https://doi.org/10.1016/j.matchemphys.2016.08.043
Langreth DC, Mehl MJ (1983) Beyond the local-density approximation in calculations of ground-state electronic properties. Phys Rev B 28:1809. https://doi.org/10.1103/PhysRevB.28.1809
Sousa SF, Fernandes PA, Ramos MJ (2007) General performance of density functionals. J Phys Chem A 111:10439–10452. https://doi.org/10.1021/jp0734474
Becke AD (1992) Density-functional thermochemistry. II. The effect of the Perdew-Wang generalized gradient correlation correction. J Chem Phys 97:9173. https://doi.org/10.1063/1.463343
Datta D, Li J, Koratkar N, Shenoy VB (2014) Enhanced lithiation in defective graphene. Carbon 80:305–310. https://doi.org/10.1016/j.carbon.2014.08.068
Fan K, Tang J, Wu S, Yang C, Hao J (2017) Adsorption and diffusion of lithium in a graphene/blue-phosphorus heterostructure and the effect of an external electric field. Phys Chem Chem Phys 19:267–275. https://doi.org/10.1039/C6CP05983J
Fu CC, Willaime F, Ordejón P (2004) Stability and mobility of mono- and di-interstitials in alpha-Fe. Phys Rev Lett 92:175503. https://doi.org/10.1103/PhysRevLett.92.175503
Zheng J, Ren Z, Guo P, Fang L, Fan J (2011) Diffusion of Li+ ion on graphene: a DFT study. Appl Surf Sci 258:1651–1655. https://doi.org/10.1016/j.apsusc.2011.09.007
Guo GC, Wang D, Wei XL, Zhang Q, Liu H, Lau WM, Liu LM (2015) First-principles study of phosphorene and graphene heterostructure as anode materials for rechargeable Li batteries. J Phys Chem Lett 6:5002–5008. https://doi.org/10.1021/acs.jpclett.5b02513
Kent P, Xie Y, Zhuang H, Dall'Agnese Y, Naguib M, Barsoum M, Gogotsi Y (2015) Prediction and characterization of MXenes for non-lithium ion battery anodes. ACS Nano 8:9606–9615. https://doi.org/10.1021/nn503921j
Funding
This work was financially supported by the Scientific Research Foundation of Sichuan Provincial Education Department (17ZA0338), Foundation of Jiangsu Institute of Marine Resources Development (JSIMR201606), and Research Start-up Foundation for Advanced Talents of Jiangsu Ocean University (KQ19021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, K., Tang, J. & Sun, Q. Monolayer Mo2C as anodes for magnesium-ion batteries. J Mol Model 26, 86 (2020). https://doi.org/10.1007/s00894-020-4347-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4347-2