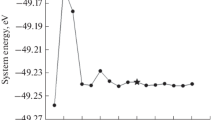
Abstract
The development of transferable interatomic potentials for the diffusion of hydrogen on palladium surfaces can be of significant value for performing molecular simulations. These molecular simulations can, in turn, lead to a better understanding of palladium-hydrogen interactions at the atomic scale. Here, we have built upon previous work to develop an analytical palladium-hydrogen-embedded atom method (EAM) potential to better describe the potential energy surface for hydrogen on palladium surfaces. This EAM potential reproduces minima and transition states calculated with density functional theory for hydrogen on Pd(111) and Pd(110) surfaces. Additionally, this potential was tested by simulating the long timescale dynamics of hydrogen adsorbed on Pd(111). Our simulations show a barrier of ca. 0.49 eV for hydrogen diffusion into the bulk of Pd(111), which is consistent with experimental results.




Similar content being viewed by others
References
Povel R, Feucht K, Gelse W, Withalm G (1989) Hydrogen fuel for motorcars. Interdiscip Sci Rev 14(4):365–373
Knapton AG (1977) Palladium alloys for hydrogen diffusion membranes. Platin Met Rev 21(2):44–50
Adams BD, Chen A (2011) The role of palladium in a hydrogen economy. Mater Today 14(6):282–289
Gdowski GE, Felter TE, Stullen RH (1987) Effect of surface temperature on the sorption of hydrogen by Pd(111). Surf Sci 181:147–155
Okuyama H, Siga W, Takagi N, Nishijima M, Aruga T (1998) Path and mechanism of hydrogen absorption at Pd(100). Surf Sci 401(3):344–354
Behm RJ, Penka V, Cattania M-G, Christmann K, Ertl G (1983) Evidence for ‘“subsurface”’ hydrogen on Pd(110): an intermediate between chemisorbed and dissolved species. J Chem Phys 78(12):7486–7490
Kay BD, Peden CHF, Goodman DW (1986) Kinetics of hydrogen absorption by Pd(110). Phys Rev B 34(2):817–822
Daw MS, Baskes MI (1984) Embedded-atom method: derivation and application to impurities, surfaces, and other defects in metals. Phys Rev B 29(12):6443–6453
Daw MS, Foiles SM, Baskes MI (1993) The embedded-atom method : a review of theory and applications. Mater Sci Reports 9(June 1992):251–310
Senftle TP, Janik MJ, Van Duin ACT (2014) A ReaxFF investigation of hydride formation in palladium nanoclusters via Monte Carlo and molecular dynamics simulations. J Phys Chem C 118(9):4967–4981
Zhou XW, Zimmerman JA, Wong BM, Hoyt JJ (2008) An embedded-atom method interatomic potential for Pd-H alloys. J Mater Res 23(3):704–718
Foiles SM, Hoyt JJ (2001) Computer simulation of bubble growth in metals due to he. Sandia Report SAND2001-0661
Zimmerman JA, Zhou XW, Griffin J, Wong BM, Hoyt JJ (2007) Development of an inter-atomic potential for the Pd-H binary system. Sandia Report SAND2007-WXYZ
Park YH, Hijazi I (2017) Development of physics based analytical interatomic potential for palladium-hydride. J Mol Model 23(4):1–11
Hijazi I, Zhang Y, Fuller R (2018) A simple embedded atom potential for Pd-H alloys. Mol Simul 44(17):1371–1379
Lynch JF, Flanagan TB (1973) An investigation of the dynamic equilibrium between chemisorbed and absorbed hydrogen in the palladium/hydrogen system. J Phys Chem 77(22):2628–2634
Plimpton S (1997) Short-range molecular dynamics. J Comput Phys 117(6):1–42
Kresse G, Hafner J (1993) Ab initio molecular dynamcis for liquid metals. Phys Rev B 47(1):558
Kresse G, Hafner J (1994) Liquid-metal—amorphous-semiconductorab initio molecular-dynamics simulations of the liquid-metal-amorphous-semiconductor transition in germanium. Phys Rev B 49(20):14251
Kresse G, Furthmiiller J (1996) Efficiency of Ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. J Am Water Works Assoc 6:15–50
Kresse G, Furthmüller J, Li YJ, Chen YJ, Walmsley JC, Mathinsen RH, Dumoulin S, Roven HJ, Yip S, Supervisor T et al (1996) Efficient iteritive schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186
Kresse G, Joubert D (1999) Kresse, Joubert - unknown - from ultrasoft pseudopotentials to the projector augmented-wave method 59(3):11–19
Blochl PE (1994) Projector augmented-wave method. Phys Rev B 50(24):17953–17979
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple - the PBE functional. Phys Rev Lett 77(18):3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Generalized gradient approximation made simple. Phys Rev Lett 78:1396
Methfessel M, Paxton AT (1989) High-precision sampling for Brillouin-zone integration in metals. Phys Rev B 40(6):3616–3621
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192
Xu L, Henkelman G (2008) Adaptive kinetic Monte Carlo for first-principles accelerated dynamics. J Chem Phys 129(11)
Henkelman G, Jónsson H (2001) Long time scale kinetic Monte Carlo simulations without lattice approximation and predefined event table. J Chem Phys 115(21):9657–9666
Chill ST, Welborn M, Terrell R, Zhang L, Berthet JC, Pedersen A, Jónsson H, Henkelman G (2014) EON: Software for long time simulations of atomic scale systems. Model Simul Mater Sci Eng 22(5)
Henkelman G, Jónsson H (1999) A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys 111(15):7010–7022
Novotny MA (1995) Monte Carlo algorithms with absorbing Markov chains: fast local algorithms for slow dynamics. Phys Rev Lett 74(1):1–5
Lischka M, Groß A (2003) Hydrogen on palladium: a model system for the interaction of atoms and molecules with metal surfaces. Recent Dev Vac Sci Technol 661(2):111–132
Bae CS, Freeman DL, Doll JD, Kresse G, Hafner J (2000) Energetics of hydrogen chemisorbed on cu(110): a first principles calculations study. J Chem Phys 113(16):6926–6932
Doye JPK, Miller MA, Wales DJ (1999) Evolution of the potential energy surface with size for Lennard-Jones clusters. J Chem Phys 111(18):8417–8428
Evans DA, Wales DJ (2003) Free energy landscapes of model peptides and proteins. J Chem Phys 118(8):3891–3897
Wales DJ, Miller MA, Walsh TR (1998) Archetypal energy landscapes. Nature 394(6695):758–760
Becker OM, Karplus M (1997) The topology of multidimensional potential energy surfaces: theory and application to peptide structure and kinetics. J Chem Phys 106(4):1495–1517
Namba K, Ogura S, Ohno S, Di W, Kato K, Wilde M, Pletikosic I, Pervan P, Milun M, Fukutani K (2018) Acceleration of hydrogen absorption by palladium through surface alloying with gold. Proc Natl Acad Sci U S A 115(31):7896–7900
Auer W, Grabke HJ (1974) The kinetics of hydrogen absorption in palladium (α- and β-phase) and palladium-silver-alloys. Ber Bunsenges Phys Chem 78(1):58–67
Funding
National Science Foundation (US) (CHE-1764230) and Welch Foundation (F-1841).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ciufo, R.A., Henkelman, G. Embedded atom method potential for hydrogen on palladium surfaces. J Mol Model 26, 336 (2020). https://doi.org/10.1007/s00894-020-04588-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04588-x