Abstract
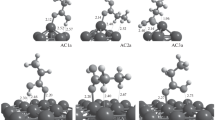
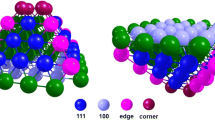
Palladium nanocatalysts are widely used in a number of industrially relevant hydrogenation reactions. Understanding the processes that occur at the surface of the catalysts during these reactions is a problem of high scientific and industrial importance. This work is devoted to the theoretical study of atomic and electronic structure of palladium (111) and (100) surfaces, and the structures of a series of hydrocarbon, which are potential intermediates of ethylene and acetylene hydrogenation/dehydrogenation reactions. It was found that in (111) surface after geometry relaxation, interplanar distances at the surface are bigger than those in the bulk, while the opposite effect is observed for (100) surface. It was shows that the above effect is reproduced in both Projector Augmented Wave method and with using Slater Type Orbitals. The effect is stronger in Generalized Gradient Approximation than in Local Density Approximation. On the obtained surfaces, 11 hydrocarbon molecules and radicals were relaxed and the probably of their formation at different surfaces was estimated. The obtained results provide important insights on the fundamental processes of hydrocarbon adsorption on palladium nanoparticles.








Similar content being viewed by others
REFERENCES
Handbook of Heterogeneous Catalysis, Ed. by H. Arnold, F. Dobert, et al. (Wiley, Weinheim, 1997).
P. Sabatier, “The Method of Direct Hydrogenation by Catalysis: Nobel Lecture, December 11, 1912,” in Nobel Lectures in Chemistry 1901–1921 (World Sci., 1999).
F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sorensen, C. H. Christensen, and J. K. Norskov, Angew. Chem., Int. Ed. 120, 9439 (2008). https://doi.org/10.1002/ange.200802844
A. A. Skorynina, A. A. Tereshchenko, O. A. Usoltsev, A. L. Bugaev, K. A. Lomachenko, A. A. Guda, E. Groppo, R. Pellegrini, C. Lamberti, and A. V. Soldatov, Radiat. Phys. Chem. 175, 108079 (2020). https://doi.org/10.1016/j.radphyschem.2018.11.033
A. L. Bugaev, O. A. Usoltsev, A. A. Guda, K. A. Lomachenko, M. Brunelli, E. Groppo, R. Pellegrini, A. V. Soldatov, and J. van Bokhoven, Faraday Discuss. 229, 197 (2021). https://doi.org/10.1039/c9fd00139e
E. G. Kamyshova, A. A. Skorynina, A. L. Bugaev, C. Lamberti, and A. V. Soldatov, Radiat. Phys. Chem. 175, 108144 (2020). https://doi.org/10.1016/j.radphyschem.2019.02.003
M. V. Kirichkov, A. L. Bugaev, A. A. Skorynina, V. V. Butova, A. P. Budnyk, A. A. Guda, A. L. Trigub, and A. V. Soldatov, Metals 10, 810 (2020). https://doi.org/10.3390/met10060810
A. L. Bugaev, A. A. Guda, I. A. Pankin, E. Groppo, R. Pellegrini, A. Longo, A. V. Soldatov, and C. Lamberti, Catal. Today 336, 40 (2019). https://doi.org/10.1016/j.cattod.2019.02.068
A. Ruban, B. Hammer, P. Stoltze, H. L. Skriver, and J. K. Norskov, J. Mol. Catal. A: Chem. 115, 421 (1997). https://doi.org/10.1016/S1381-1169(96)00348-2
H. Gruber-Woelfler, P. F. Radaschitz, P. W. Feenstra, W. Haas, and J. G. Khinast, J. Catal. 286, 30 (2012). https://doi.org/10.1016/j.jcat.2011.10.013
A. Sárkány, A. Horváth, and A. Beck, Appl. Catal. 229, 117 (2002). https://doi.org/10.1016/S0926-860X(02)00020-0
F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sorensen, C. H. Christensen, and J. K. Norskov, Science 320 (5881), 1320 (2008). https://doi.org/10.1126/science.1156660
F. Mittendorfer, C. Thomazeau, P. Raybaud, and H. Toulhoat, J. Phys. Chem. B 107, 12287 2003). https://doi.org/10.1021/jp035660f
P. Sautet and J.-F. Paul, Catal. Lett. 9, 245 (1991).
J. L. Davis and M. A. Barteau, J. Am. Chem. Soc. 111, 1782 (1989). https://doi.org/10.1021/ja00187a035
I. Alkorta and J. Elguero, Int. J. Mol. Sci. 4, 64 (2003). https://doi.org/10.3390/i4030064
A. D. Laurent and D. Jacquemin, Int. J. Quantum Chem. 113, 2019 (2013). https://doi.org/10.1002/qua.24438
G. R. Schleder, A. C. M. Padilha, C. M. Acosta, M. Costa, and A. Fazzio, J. Phys.: Mater. 2, 032001 (2019). https://doi.org/10.1088/2515-7639/ab084b
G. de Velde, F. M. Bickelhaupt, E. J. Baerends, Guerra C. Fonseca, S. J. van Gisbergen, J. G. Snijders, and T. Ziegler, J. Comput. Chem. 22, 931 (2001). https://doi.org/10.1002/jcc.1056
G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys. 54, 11169 (1996). https://doi.org/10.1103/PhysRevB.54.11169
G. Kresse and J. Furthmüller, Comput. Mater. Sci. 6, 15 (1996). https://doi.org/10.1016/0927-0256(96)00008-0
G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys. 59, 1758 (1999). https://doi.org/10.1103/PhysRevB.59.1758
A. L. Bugaev, M. Zabilskiy, A. A. Skorynina, O. A. Usoltsev, A. V. Soldatov, and J. A. van Bokhoven, Chem. Commun. 56, 13097 (2020). https://doi.org/10.1039/D0CC05050D
A. L. Bugaev, O. A. Usoltsev, A. A. Guda, K. A. Lomachenko, I. A. Pankin, Y. V. Rusalev, H. Emerich, E. Groppo, R. Pellegrini, A. V. Soldatov, J. A. van Bokhoven, and C. Lamberti, J. Phys. Chem. C 122, 12029 (2018). https://doi.org/10.1021/acs.jpcc.7b11473
O. A. Usoltsev, A. Y. Pnevskaya, E. G. Kamyshova, A. A. Tereshchenko, A. A. Skorynina, W. Zhang, T. Yao, A. L. Bugaev, and A. V. Soldatov, Nanomaterials 10, 1643 (2020). https://doi.org/10.3390/nano10091643
O. A. Usoltsev, A. L. Bugaev, A. A. Guda, S. A. Guda, and A. V. Soldatov, Top. Catal. 63, 58 (2020). https://doi.org/10.1007/s11244-020-01221-2
F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sorensen, C. H. Christensen, and J. K. Norskov, Angew. Chem., Int. Ed. Engl. 47, 9299 (2008). https://doi.org/10.1002/anie.200802844
D. Teschner, Z. Revay, J. Borsodi, M. Havecker, A. Knop-Gericke, R. Schlogl, D. Milroy, S. D. Jackson, D. Torres, and P. Sautet, Angew. Chem., Int. Ed. Engl. 47, 9274 (2008). https://doi.org/10.1002/anie.200802134
H. Kubicka, J. Catal. 12, 223 (1968). https://doi.org/10.1016/0021-9517(68)90102-4
S. Krüger, S. Vent, F. Nortemann, M. Staufer, and N. Rosch, J. Chem. Phys. 115, 2082 (2001). https://doi.org/10.1063/1.1383985
I. V. Yudanov, K. M. Neyman, and N. Rosch, Phys. Chem. Chem. Phys. 6, 116 (2004). https://doi.org/10.1039/b311054k
J. Paier, R. Hirschl, M. Marsman, and G. Kresse, J. Chem. Phys. 122, 234102 (2005). https://doi.org/10.1063/1.1926272
J. Paier, M. Marsman, K. Hummer, G. Kresse, I. C. Gerber, and J. G. Angyan, J. Chem. Phys. 124, 154709 (2006). https://doi.org/10.1063/1.2187006
J. P. Perdew, Phys. Rev. Lett. 55, 1665 (1985). https://doi.org/10.1103/PhysRevLett.55.1665
R. A. Evarestov and V. P. Smirnov, Phys. Rev. B: Condens. Matter Mater. Phys. 70, 233101 (2004). https://doi.org/10.1103/PhysRevB.70.233101
J. D. Pack and H. J. Monkhorst, Phys. Rev. B: Solid State 16, 1748 (1977). https://doi.org/10.1103/PhysRevB.13.5188
J. R. Shewchuk, An Introduction to the Conjugate Gradient Method without the Agonizing Pain (Carnegie-Mellon Univ., Pittsburgh, 1994).
T. Steihaug, SIAM J. Numer. Anal. 20, 626 (1983). https://doi.org/10.1137/0720042
FUNDING
The study was carried out with financial support from the Ministry of Science and Higher Education of the Russian Federation within the framework of the state assignment in the science field no. 0852-2020-0019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pnevskaya, A.Y., Kozyr, E.G., Al-Jaf, B.J. et al. First Principal Simulation of Palladium Nanocatalysts Surfaces. J. Surf. Investig. 15, 1270–1277 (2021). https://doi.org/10.1134/S102745102106015X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102745102106015X