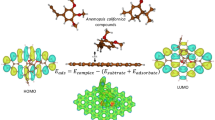
Abstract
Detection and qualification process related to impurities assume importance in pharmacological drug development programs, and the present article gives the structural and spectral characterization of sulfone derivatives their self-assembly with graphene sheets theoretically. The investigation of adsorption behavior of sulfone compounds can provide valuable information about its reactivity and electronic and structural properties. Three-dimensional electrostatic potential diagrams were mapped. The frontier orbital energies and energy bandgaps of the molecules were computed. Delocalization of charge density between the bonding or lone pair and antibonding orbitals calculated by NBO analysis. Docking was executed to investigate binding areas of chemical compounds. Bioactivity scores show that the pharmacokinetic and pharmacological properties of the ligands are appropriate leading to be considered potential drug agents. The obtained theoretical wavenumber results of the present study were fully compatible with the experimental results.
Graphical abstract


Similar content being viewed by others
References
N’dri JS, Kablan ALC, Ouattara B, Kone MG, Ouattara L, Kodjom CG, Ziao N (2019) QSAR studies .of the antifungal activities of alpha-diaminophosphonates derived from dapsone by DFT method. J Mater Phys Chem 7:1–7
Paniker U, Levine N (2001) Dapsone and sulfapyridine. Dermatol Clin 19:79–86
Chiodini P (1987) The chemoprophylaxis of malaria. J Antimicrob Chemother 20:297–302
Powell R, DeGowin R, Eppes R, McNamara J, Carson P (1967) The antimalarial and hemolytic properties of 4,4′-diaminodiphenylsulfone (DDS). Int J Lepr Other Mycobact Dis 35:590–604
Lee B, Medina I, Benowitz N (1989) Dapsone, trimethoprim and sulfamethoxazole plasma levels during treatment of Pneumocystic carinii pneumonia in patients with the acquired immunodeficiency syndrome (AIDS): evidence of drug interactions. Ann Intern Med 110:606–611
Hughes W, Smith B (1984) Efficacy of diaminodiphenylsulfone and other drugs in murine penumocystis carinii pneumonitis. Antimicrob Agents Chemother 26:436–440
Leoung GS, Mills J, Hopewell PC, Hughes W, Wofsy C (1986) Dapsone-trimethoprim for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Ann Intern Med 105:45–48
Zhu YI, Stiller MI (2001) Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 45:420–434
Wallis L, Gilson RC, Gilson RT (2018) Dapsone for recalcitrant eosinophilic annular erythema: a case report and literature review. Dermatol Ther 8:157–163
Liang SE, Hoffman R, Peterson E, Soter NA (2019) Use of dapsone in the treatment of chronic idiopathic and autoimmune urticaria. JAMA Dermatol 155:90–95
Moura SL, Fernandes GFS, Machdo FBC, Ferrao LFA (2020) Theoretical and experimental electronic spectra of neutral, monoprotonated and diprotonated dapsone. Theor Chem Accounts 139:53
Moura SL, Santos JR, Machado FBC, Kawachi EY, Gerrao LFA (2015) Conductive organic polymers: an electrochemical route for the polymerization of dapsone. J Electroanal Chem 757:230–234
Chakravarthy MP, Mohana KN, Kumar CBP, Badiea AM (2015) Corrosion inhibition behavior and adsorption characteristics of dapsone derivatives on mild steel in acid medium. Am Chem Sci 8:1–16
Ravichandran P, Athinarayanan J, Premnath D, Periasamy VS, Alshatwi AA, Vasanthkumar S (2015) Synthesis, molecular docking and biological evaluation of novel 6-(4-(4-aminophenylsulfonyl)phenylamino)-5H-benzo[1]phenothiazin-5-one derivatives. Spectrochim Acta 139:477–487
Fares M, Said MA, Alsherbiny MA, Eladwy RA, Almahli H, Abdel-Aziz MM, Ghabbour HA, Eldehna WM, Abdel-Aziz HA (2016) Synthesis, biological evaluation and molecular docking of certain sulfones as potential nonazole antifungal agents. Molecules 21:114
Konduru NK, Dey S, Sajid M, Owais M, Ahmed N (2013) Synthesis and antibacterial and antifungal evaluation of chalcone based sulfones and bisulfones. Eur J Med Chem 59:23–30
Ildiz GO, Akyuz S (2012) Conformational analysis and vibrational spectroscopic studies on dapsone. Opt Spectrosc 113:495–504
Chandran A, Mary YS, Varghese HT, Panicker CY, Pazdera P, Rajendran G, Babu N (2011) FT-IR, FT-Raman spectroscopy and computational study of N-carbamimidoyl-4-{[(E)-((2-hydroxyphenyl)methylidene]amino}benzenesulfonamide. J Mol Struct 992:77–83
Chandran A, Varghese HT, Mary YS, Panicker CY, Manojkumar TK, Van Alsenoy C, Rajendran G (2012) FT-IR, FT-Raman and computational study of (E)-N-carbamimidoyl-4-((4-methoxybenzylidene)amino)benzenefulfonamide. Spectrochim Acta 92:84–90
Mary YS, Raju K, Yildiz I, Temiz-Arpaci O, Nogueira HIS, Granadeiro CM, Van Alsenoy C (2012) FT-IR, FT-Raman, SERS and computational study of 5-ethylsulphonyl-2-(o-chlorobenzyl)benzoxazole. Spectrochim Acta 96:617–625
Chandran A, Varghese HT, Mary YS, Panicker CY, Manojkumar TK, Van Alsenoy C, Rajendran G (2012) Vibrational spectroscopic and quantum chemical calculations of (E)-N-carbamimidoyl-4-((naphthalene-1-yl-methylene)amino)benzene sulfonamide. Spectrochim Acta 87:29–39
Chandran A, Mary YS, Varghese HT, Panicker CY, Manojkumar TK, Van Alsenoy C (2012) Rajendran G (2012) vibrational spectroscopic study of (E)-4-(benzylideneamino)-N-carbamimidoyl benzene sulfonamide. ISRN Anal Chem. https://doi.org/10.5402/2012/397026
Kostarelos K (2003) Rational design and engineering of delivery systems for therapeutics: biomedical exercises in colloid and surface science. Adv Colloid Interf Sci 106:147–168
Vanesa SC, Jachak A, Hurt RH, Kane BA (2012) Biological interactions of graphene family nano materials: an interdisciplinary review. Chem Res Toxicol 25:15–34
Hao Q, Wang B, Bossar JA, Kiraly B, Zeng Y, Ching IK, Jensen L, Werner DH, Huang TJ (2012) Surface enhanced Raman scattering study on graphene coated metallic nano structure substrates. J Phys Chem C 116:7249–7254
Zhu X, Shi L, Schmidt MS, Boisen A, Hansen O, Zi J, Xiao S, Mortensen NA (2013) Enhanced light matter interactions in graphene covered gold nanovoid arrays. Nano Lett 13:4690–4696
Osvath Z, Deak A, Kertesz K, Molnar G, Vertesy G, Zamb D, Hwng C, Biro LP (2015) The structure and properties of graphene on gold nanoparticles. Nanoscale 7:5503–5509
Ali M, Tit N, Pi XD, Yamani ZH (2019) First principles study on the functionalization of graphene with Fe catalyst for the detection of CO2: effect of catalyst clustering. Appl Surf Sci 502:144153
Santosh R, Kumar V (2019) The structural, electronic, optical and thermodynamical properties of hydrofluorinated graphene: first principle calculations. Sold State Sci 94:70–76
Sharma V, Kagdada HL, Wang JL, Jha PK (2019) Hydrogen adsorption on pristine and platinum decorated graphene quantum dot, a first principle study. Int J Hydrog Energy 45:23977–23987
Mihalache I, Radoi A, Mihaila M, Munteanu C, Marin A, Danila M, Kusko M, Kusko C (2015) Charge and energy transfer interplay in hybrid sensitized solar cells mediated by graphene quantum dots. Electrochim Acta 153:306–315
Li Y, Li X, Xu Y (2020) Theoretical insights into the effect of pristine, doped and hole graphene on the overall performance of dye sensitized solar cells. Inorg Chem Front 7:157
Ni J, Quintana M, Song S (2020) Adsorption of small gas molecules on transition metal (Fe, Ni and co, cu) doped graphene : a systematic DFT study. Phys E Low Dimension Syst Nanostruct 116:113768
Dastani N, Arab A, Raissi H (2020) DFT computational study towards investigating cladribine anticancer drug adsorption on the graphene and functionalized graphene. Struct Chem. https://doi.org/10.1007/s11224-020-01526-8
Zhu H, Xu Z, Xie D, Fang Y (2018) Graphene: fabrication, characterizations, properties and applications. Academic Press, London
Nicolai A, Sumpter BG, Meunier V (2014) Tunaboe water desalination across graphene oxide framework membranes. Phys Chem Chem Phys 16:8646–8654
Martin MJ, May S, Mebberson N, Pendleton P, Vasileve K, Plush SE, Hayball JD (2017) Activted carbon, carbon nanotubes and graphene: materials and composites for advanced water purification. J Carbon Res 3:1–29
Reddy AVB, Moniruzzaman M, Reddy YVM, Madhavi G (2019) Graphene-based nanomaterials for the removal of pharmaceuticals in drinking water sources, in graphene-based nanotechnologies for energy and environmental applications. Pp. 329-358 Elsevier
Al-Jumaili A, Alancherry S, Bazaka K, Jacob M (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10:1066
Ahamadi R, Sarvestani MRJ, Sadeghi B (2018) Computational study of the fullerene effects on the properties of 16 different drugs: a review. Int J Nano Dimens 9:325–335
Sun Q, Zhang R, Qiu J, Liu R, Xu W (2018) On-surface synthesis of carbon nanostructures. Adv Mater 30:1705630
Jiang Y, Wang J, Malfatti L, Carboni D, Senes N, Innocenzi P (2018) Highly durable graphene mediated surface enhanced Raman scattering (G-SERS) nanocomposites for molecular detection. Appl Surf Sci 450:451–460
Becke AD (1993) Density functional thermo chemistry. III The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev B37:785–789
Gaussian 09, Revision B.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian, HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli, C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian, Inc., Wallingford CT
Dennington RD, Keith TA, Millam JM (2008) GaussView, Gaussian Inc
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Zhao JY, Zhang Y, Zhu LG (2004) Theoretical studies on vibrational spectra of mixed cyanide-halide complexes of gold(III). J Mol Struct Theochem 671:179–187
Foresman JB (1996) In: Frisch E (ed) Exploring chemistry with electronic structure methods: a guide to using Gaussian. Gaussian Inc, Pittsburg
Al-Otaibi JS, Mary YS, Mary YS, Thomas R (2019) Quantum mechanical and photovoltaic studies on the cocrystals of hydrochlorothiazide with isonazid and malonamide. J Mol Struct 1197:719–726
Bio-Rad Laboratories Inc SpectraBase; http://spectrabase.com/. Accessed 25 Sept 2019
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Kaur M, Mary YS, Varghese HT, Panicker CY, Yathirajan HS, Siddegowda MS, Van Alsenoy C (2012) Vibrational spectroscopic, molecular structure, first hyperpolarizability and NBO studies of 4′-methylphenyl-2-carbonitrile. Spectrochim Acta 98:91–99
Shafieyoon P, Mehdipour E, Mary YS (2019) Synthesis, characterization and biological investigation of glycine based sulfonamide derivative and its complex: vibration assignment, HOMO-LUMO analysis, MEP and molecular docking. J Mol Struct 1181:244–252
Mary YS, Miniyar PB, Mary YS, Resmi KS, Panicker CY, Armakovic S, Armakovic SJ, Thomas R, Sureshkumar B (2018) Synthesis and spectroscopic study of three new oxadiazole derivatives with detailed computational evaluation of their reactivity and pharmaceutical potential. J Mol Struct 1173:469–480
Mary YS, Mary YS, Thomas R, Narayana B, Samshuddin S, Sarojini BK, Armakovic S, Armakovic SJ, Pillai GG (2019) Theoretical studies on the structure and various physic-chemical and biological properties of a terphenyl derivative with immense anti-protozoan activity. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2019.1624974
Mary YS, Varghese HT, Panicker CY, Girisha M, Sagar BK, Yathirajan HS, Al-Saadi AA, Van Alsenoy C (2015) Vibrational spectra, HOMO, LUMO, NBO, MEP analysis and molecular docking study of 2,2-diphenyl-4-(piperidin-1yl)butanamide. Spectrochim Acta 150:543–556
Beegum S, Mary YS, Mary YS, Thomas R, Armakovic S, Armakovic SJ, Zitko J, Dolezal M, Van Alsenoy C (2020) Exploring the detailed spectroscopic characteristics, chemical and biological activity of two cyanopyrazine-2-carboxamide derivatives using experimental and theoretical tools. Spectrochim Acta 224:117414
Mary YS, Panicker CY, Sapnakumari M, Narayana B, Sarojini BK, Al-Saadi AA, Van Alsenoy C, War JA, Fun HK (2015) Molecular structure, FT-IR, vibrational assignments, HOMO-LUMO analysis and molecular docking study of 1-[5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Spectrochim Acta 136:473–482
Sureshkumar B, Mary YS, Panicker CY, Suma S, Armakovic S, Armakovic SJ, Van Alsenoy C, Narayna B (2020) Quinoline derivatives as possible lead compounds for anti-malarial drugs: spectroscopic, DFT and MD study. Arab J Chem 13:632–648
Zhang T, Wei X, Zuo Y, Chao J (2019) An efficient measure to improve NLO performance by point charge electric field. Optik 182:295–302
Sheeja SR, Mangalam NA, Kurup MRP, Mary YS, Raju K, Varghese HT, Panicker CY (2010) Vibrational spectroscopic studies and computational study of quinoline-2-carbaldehyde benzoyl hydrazone. J Mol Struct 973:36–46
Al-Otaibi JS, Mary YS, Mary YS, Panicker CY, Thomas R (2019) Cocrystals of pyrazinamide with p-toluenesulfonic and ferulic acids: DFT investigations and molecular docking studies. J Mol Struct 1175:916–926
Thomas R, Mary YS, Resmi KS, Narayana B, Sarojini BK, Vijayakumar G, Van Alsenoy C (2019) Two neoteric pyrazole compounds as potential anti-cancer agents:synthesis, electronic structure, physico-chemical properties and docking analysis. J Mol Struct 1181:455–466
Mary YS, Ertan-Bolelli T, Thomas R, Krishnan AR, Bolelli K, Kasap EN, Onkol T, Yildiz I (2019) Quantum mechanical studies of three aromatic halogen-substituted bioactive sulfonamidobenzoxazole compounds with potential light harvesting properties. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2019.1689405
Mary YS, Yalcin G, Mary YS, Resmi KS, Thomas R, Önkol T, Kasap EN, Yildiz I (2020) Spectroscopic, quantum mechanical studies, ligand protein interactions and photovoltaic efficiency modeling of some bioactive benzothiazolinone acetamide analogs. Chem Pap 74:1957–1964
Mary YS, Mary YS, Resmi KS, Kumar VS, Thomas R, Sureshkumar B (2019) Detailed quantum mechanical, molecular docking, QSAR prediction, photovoltaic light harvesting effficiency analysis of benzil and its halogenated analogues. Heliyon 5:e02825
Roeges NPG (1994) A guide to the complete interpretation of infrared spectra of organic structures. Wiley Inc, New York
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166
Albrecht MG, Creighton JA (1977) Anomalously intense Raman spectra of pyridine at a silver electrode. J Am Chem Soc 99:5215–5217
Jeanmaire DL, Van duyne RP (2006) Surface Raman spectro electrochemistry. J Electroanal Chem Interfacial Electrochem 84:1–20
Smith E, Dent G (2005) Modern Raman spectroscopy – a practical approach. https://doi.org/10.1002/0470011831
Kneipp K, Wang Y, Kneipp H, Perelman LT, Itzkan I, Dasari RR, Feld MS (1997) Single molecule detection using surface enhanced Raman scattering (SERS). Phys Rev Lett 78:1667–1670
Nie S, Emory SR (1997) Probing single molecules and single nano particles by surface enhanced Raman scattering. Science 275:1102–1106
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field in atomically thin carbon films. Science 306:666–669
Sharma V, Som NN, Pillai SB, Jha PK (2020) Utilization of doped GQDs for ultrasensitive detection of catastrophic melamine: a new SERS platform. Spectrochim Acta 224:117352
Al-Otaibi JS (2020) Detailed quantum mechanical studies on bioactive benzodiazepine derivatives and their adsorption over graphene sheets. Spectrochim Acta 235:118333
Al-Otaibi JS, Almuqrin AH, Mary YS, Mary YS (2020) Comprehensive quantum mechanical studies on three bioactive anastrozole based triazole analogues and their SERS active graphene complex. J Mol Struct 1217:128388
Lagunin A, Stepanchikova A, Filimonov D, Poroikov V (2000) PASS: prediction of activity spectra for biologically active substances. Bioinformatics 16:747–748
Hevener KE, Yun MK, Qi J, Kerr ID, Babaoglu K, Hurdel JG, Balakrishnan K, White SW, Lee RE (2010) Structural studies of pterin based inhibitors of dihydropteroate synthase. J Med Chem 53:166–177
Trott O, Olson AJ (2010) Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Kramer B, Rarey M, Lengauer T (1999) Evaluation of the FlexX incremental construction algorithm for protein ligand docking. Proteins Struct Funct Genet 37:228–241
Haress NG, Al-Omary F, El-Emam AA, Mary YS, Panicker CY, Al-Saadi AA, War JA, Van Alsenoy C (2015) Spectroscopic investigation (FT-IR and FT-Raman), vibrational assignments, HOMO-LUMO analysis and molecular docking study of 2-(adamantan—1yl)-5-(4-nitrophenyl)-1,3,4-oxadiazole. Spectrochim Acta 135:973–983
Mary YS, Varghese HT, Panicker CY, Thiemann T, Al-Saadi AA, Popoola SA, Van Alsenoy C, Jasem YA (2015) Molecular conformational analysis, vibrational spectra, NBO, NLO, HOMO-LUMO and molecular docking studies of ethyl 3-(E)-(anthracen-9-yl)prop-2-enoate based on density functional theory calculations. Spectrochim Acta 150:533–542
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Research Funding Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Otaibi, J.S., Almuqrin, A.H., Mary, Y.S. et al. DFT and molecular docking studies of self-assembly of sulfone analogues and graphene. J Mol Model 26, 273 (2020). https://doi.org/10.1007/s00894-020-04546-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04546-7