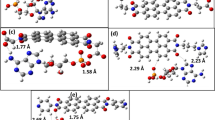
Abstract
In order to explore the essence of the hydration process of chitin or chitosan in the presence of cation, the cooperativity effects between the H-bonding and Na+···molecule interactions in the 1,4-dimethoxy-d-glucosamine (DMGA) complexes with H2O and Na+ were investigated at the B3LYP/6-311++G(d,p), M06-2X/6-311++G(2df,2p), and ωB97X-D/6-311++G(2df,2p) levels. The result shows that the complexes in which Na+ or H2O is bonded simultaneously to the –NH and –OH groups connected to the C3 atom of DMGA are the most stable. The cooperativity and anti-cooperativity effects occur in DMGA···H2O···DMGA and DMGA···Na+···H2O, while only the cooperativities are confirmed in DMGA···Na+···DMGA. The cooperativity occurs in the DMGA···Na+···H2O complexes without the hydration, while the anti-cooperativity occurs in those with the hydration. Furthermore, the cooperativity and anti-cooperativity in DMGA···Na+···H2O are far stronger than those in DMGA···Na+···DMGA or DMGA···H2O···DMGA. Therefore, a deduction is given that the cooperativity and anti-cooperativity effects play an important role in the hydration of chitin or chitosan in the presence of Na+. When only Na+ is linked with –OH and –NH groups of chitosan or chitin, due to the cooperativity effect, the hydration does not occur. When both Na+ and H2O are linked with –OH and –NH groups, the anti-cooperativities are dominant in controlling of the aggregation process of Na+, H2O, chitosan, and chitin, leading to the possible hydration. Atoms in molecules (AIM) analysis confirms the cooperativity and anti-cooperativity effects.

Graphical abstract




Similar content being viewed by others
References
Vijay D, Sastry GN (2010) The cooperativity of cation-π and π-π interactions. Chem. Phys. Lett. 485:235–242
Mahadevi AS, Sastry GN (2016) Cooperativity in noncovalent interactions. Chem. Rev. 116:2775–2825
Mignon P, Loverix S, Steyaert J, Geerlings P (2005) Influence of the π-π interaction on the hydrogen bonding capacity of stacked DNA/RNA bases. Nucl Acids Res 33:1779–1789
Hesselmann A, Jansen G, Schutz M (2006) Interaction energy contributions of H-bonded and stacked structures of the AT and GC DNA base pairs from the combined density functional theory and intermolecular perturbation theory approach. J. Am. Chem. Soc. 128:11730–11731
Leist R, Frey JA, Ottiger P, Frey HM, Leutwyler S, Bachorz RA, Klopper W (2007) Nucleobase-fluorobenzene interactions: hydrogen bonding wins over π-stacking. Angew. Chem. Int. Ed. 46:7449–7452
Hruby SL, Shanks BH (2009) Acid–base cooperativity in condensation reactions with functionalized mesoporous silica catalysts. J. Catal. 263:181–188
Varfolomeev MA, Abaidullina DI, Gainutdinova AZ, Solomonov BN (2010) FTIR study of H-bonds cooperativity in complexes of 1,2-dihydroxybenzene with proton acceptors in aprotic solvents: influence of the intramolecular hydrogen bond. Spectrochim Acta Part A 77:965–972
Garcia-Raso A, Albertí FM, Fiol JJ, Tasada A, Barceló-Oliver M, Molins E, Escudero D, Frontera A, Quiñonero D, Deyà PM (2007) Anion-π interactions in bisadenine derivatives: a combined crystallographic and theoretical study. Inorg. Chem. 46:10724–10735
Alkorta I, Blanco F, Deyà PM, Elguero J, Estarellas C, Frontera A, Quiñonero D (2010) Cooperativity in multiple unusual weak bonds. Theor. Chim. Acta 126:1–14
Hunter CA, Anderson HL (2009) What is cooperativity? Angew Chem Int Ed Engl 48:7488–7499
Ferguson A, Liu Z, Chan HS (2009) Desolvation barrier effects are a likely contributor to the remarkable diversity in the folding rates of small proteins. J. Mol. Biol. 389:619–636
Madeiraa PP, Bessaa A, Loureiro JA, Álvares-Ribeiroc L, Rodrigues AE, Zaslavsky BY (2015) Cooperativity between various types of polar solute–solvent interactions in aqueous media. J. Chromatogr. A 1408:108–117
Zaitseva KV, Varfolomeev MA, Novikov VB, Solomonov BN (2011) Enthalpy of cooperative hydrogen bonding in complexes of tertiary amines with aliphatic alcohols: calorimetric study. J Chem Thermodynamics 43:1083–1090
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31:603–632
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci. 34:641–678
Wysokowski M, Petrenko I, Stelling AL, Stawski D, Jesionowski T, Ehrlich H (2015) Poriferan chitin as a versatile template for extreme biomimetics. Polymers 7:235–265
Azizi Samir MAS, Alloin F, Dufresne A (2005) Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 6:612–626
Kim J, Yun S, Ounaies Z (2006) Discovery of cellulose as a smart material. Macromolecules 39:4202–4206
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 40:3941–3994
Lin N, Huang J, Dufresne A (2012) Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 4:3274–3294
Sinko R, Qin X, Keten S (2015) Interfacial mechanics of cellulose nanocrystals. MRS Bull. 40:340–348
Chybczyńska K, Markiewicz E, Grząbka-Zasadzińska A, Borysiak S (2019) Dielectric, magnetic, and mechanical properties of composites consisting of biopolymer chitosan matrix and hybrid spinel/cellulose filler. Ceram. Int. 45:9468–9476
Huang Y, Lapitsky Y (2013) Determining the colloidal behavior of ionically crosslinked polyelectrolytes with isothermal titration calorimetry. J. Phys. Chem. B 117:9548–9557
Cai Y, Lapitsky Y (2014) Formation and dissolution of chitosan/pyrophosphate nanoparticles: is the ionic crosslinking of chitosan reversible? Colloid Surface B 115:100–108
Palomec-Garfias AF, Jardim KV, Sousa MH, Márquez-Beltrán C (2018) Influence of polyelectrolyte chains on surface charge and magnetization of iron oxide nanostructures. Colloid Surface A 549:13–24
Patra D, Mishra P, Vijayan M, Surolia A (2016) Negative cooperativity and high affinity in chitooligosaccharide binding by a Mycobacterium smegmatis protein containing LysM and lectin domains. Biochemistry 55:49–61
Shilova SV, Tret’yakova AYA, Barabanov VP (2019) Cooperative binding of sodium dodecyl sulfate with chitosan in water-alcohol mixtures. Polym Sci Ser A 61:39–45
Mikešová J, Hašek J, Tishchenko G, Morganti P (2014) Rheological study of chitosan acetate solutions containing chitinnanofibrils. Carbohyd Polym 112:753–757
Těšínský M, Šimčíková D, Heneberg P (2019) First evidence of changes in enzyme kinetics and stability of glucokinase affected by somatic cancer-associated variations. BBA-Proteins Proteom 1867:213–218
Peña I, Kolesnikova L, Cabezas C, Bermudez C, Berdakin M, Simao A, Alonso JL (2014) The shape of D-glucosamine. Phys. Chem. Chem. Phys. 16:23244–23250
Shirahama K (1998) In: Kwak JCT (ed) Polymer-surfactant systems. Marcel Dekker, New York
Deringer VL, Englert U, Dronskowski R (2016) Nature, strength, and cooperativity of the hydrogen-bonding network in α-chitin. Biomacromolecules 17:996–1003
Deringer VL, George J, Dronskowski R, Englert U (2017) Plane-wave density functional theory meets molecular crystals: thermal ellipsoids and intermolecular interactions. Acc. Chem. Res. 50:1231–1239
Madeleine-Perdrillat C, Karbowiak T, Raya J, Gougeon R, Bodart PR, Debeaufort F (2015) Water-induced local ordering of chitosan polymer chains in thin layer films. Carbohyd Polym 118:107–114
Qiao C, Ma X, Zhang J, Jinshui Yao J (2019) Effect of hydration on water state, glass transition dynamics and crystalline structure in chitosan films. Carbohyd Polym 206:602–608
Guan L, Xu HY, Huang DH (2011) The investigation on states of water in different hydrophilic polymers by DSC and FTIR. J Poly Res 18:681–689
Madeleine-Perdrillat C, Karbowiak T, Debeaufort F, Delmotte L, Vaulot C, Champion D (2016) Effect of hydration on molecular dynamics and structure in chitosan films. Food Hydrocolloid 61:57–65
Neto CGT, Giacometti JA, Job AE, Ferreira FC, Fonseca JLC, Pereira MR (2005) Thermal analysis of chitosan based networks. Carbohyd Polym 62:97–103
Alvarado S, Sandoval G, Palos I, Tellez S, Aguirre-Loredo Y, Velazquez G (2015) The effect of relative humidity on tensile strength and water vapor permeability in chitosan, fish gelatin and transglutaminase edible films. J Food Sci Tech 35:690–695
McDonnell MT, Greeley DA, Kit KM, Keffer DJ (2016) Molecular dynamics simulations of hydration effects on solvation, diffusivity, and permeability in chitosan/chitin films. J. Phys. Chem. B 120:8997–9010
Mano JF (2008) Viscoelastic properties of chitosan with different hydration degrees as studied by dynamic mechanical analysis. Macromol. Biosci. 8:69–76
Ogawa K, Oka K, Yui T (1993) X-ray study of chitosan-transition metal complexes. Chem. Mater. 5:726–728
Okuyama K, Noguchi K, Miyazawa T, Yui T, Ogawa K (1997) Molecular and crystal structure of hydrated chitosan. Macromolecules 30:5849–5855
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 120:215–241
Mardirossian N, Head-Gordon M (2016) ωB97M-V: a combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys. 144:214110
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Inc. Wallingford CT, USA
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford
Duijineveldt FB, Duijineveldt-van de Rijdt JCMV, Lenthe JHV (1994) State of the art in counterpoise theory. Chem. Rev. 94:1873–1885
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the difference of separate total energies. Some procedures with reduced errors. Mol. Phys. 19:553–566
Yui T, Imada K, Okuyama K, Obata Y, Suzuki K, Ogawa K (1994) Molecular and crystal structure of the anhydrous form of chitosan. Macromolecules 27:7601–7605
Sikorski P, Hori R, Wada M (2009) Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 10:1100–1105
Reed AE, Curtis LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88:899–926
Gorshkova MY, Volkova IF, Izumrudov VA (2010) Nonstoichiometric polyelectrolyte complexes of chitosan soluble in neutral solutions. Polym Sci Ser A 52:368–373
Rogina A, Lončarević A, Antunović M, Marijanović I, Ivanković M, Ivanković H (2019) Tuning physicochemical and biological properties of chitosan through complexation with transition metal ions. Int. J. Biol. Macromol. 129:645–652
Terreux R, Domard M, Viton C, Domard A (2006) Interactions study between the copper II ion and constitutive elements of chitosan structure by DFT calculation. Biomacromolecules 7:31–37
Gomes JRB, Jorge M, Gomes P (2014) Interaction of chitosan and chitin with Ni, cu and Zn ions: a computational study. J. Chem. Thermodyn. 73:121–129
Murray JS, Politzer P (2011) The electrostatic potential: an overview. WIREs Comput Mol Sci 1:153–163
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol. Phys. 115:2315–2372
Jiang L, Bai P, Ren F, Wang J, Liu B, Li Y (2020) Theoretical evaluation to improve the performance of composite wax powder: cooperativity effects involving the strong Na+···π/σ and weak hydrogen-bonding interactions in the complex of graphene oxide with Na+ and CH4. Mol Phys 118, In press. https://doi.org/10.1080/00268976.2019.1612106
Domard A (1986) pH and c.d. measurements on a fully deacetylated chitosan: application to CuII-polymer interactions. Int. J. Biol. Macromol. 9:98–104
Schlick SH (1986) Binding sites of Cu2+ in chitin and chitosan. An electron spin resonance study. Macromolecules 19:192–195
Escudero D, Frontera A, Quiñonero D, Deyà PM (2008) Interplay between cation–π and H-bonding interactions. Chem. Phys. Lett. 456:257–261
Yan Y, Shi W, Ren F, Wang Y (2012) A B3LYP and MP2(full) theoretical investigation on the cooperativity effect between cation-molecule and hydrogen-bonding interactions in the O-cresol complex with Na+. Comput Theor Chem 996:91–102
Zhao GM, Liu YC, Shi WJ, Chai T, Qin SD (2014) A theoretical investigation into the cooperativity effect involving anionic hydrogen bond, thermodynamic property and aromaticity in Cl···benzonitrile···H2O ternary complex. Comput Theor Chem 1035:76–83
Zabardasti A, Zare N, Arabpour M (2011) Theoretical study of dihydrogen bonded clusters of water with tetrahydroborate. Structl Chem 22:691–695
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
We allow the journal to review all the data, and we confirm the validity of results. There is none as regards financial relationships. This work was not published previously and it is not submitted to more than one journal. It is also not split up into several parts for submission. No data have been fabricated or manipulated.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Interaction energies of the binary systems DMGA···H2O, DMGA···Na+, Na+···H2O and DMGA···DMGA at three levels of theory, the optimized geometries of the binary systems DMGA···DMGA, DMGA···H2O, DMGA···Na+ and ternary systems DMGA···H2O···DMGA, DMGA···Na+···DMGA, DMGA···Na+···H2O as well as their AIM results at the B3LYP/6-311++G(d,p) level are collected in Supplementary data. (DOC 6110 kb)
Rights and permissions
About this article
Cite this article
Zhao, Ja., Ren, Fd. Theoretical investigation into the cooperativity effect of 1,4-dimethoxy-d-glucosamine complex with Na+ and H2O. J Mol Model 26, 203 (2020). https://doi.org/10.1007/s00894-020-04461-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04461-x