Abstract
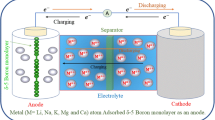
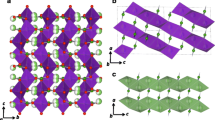
In this research, borazine-doped nanographene (BNG) decorated with halides as the anode material for Li-ion batteries (LIBs) has been investigated by means of first-principles calculations. The calculated adsorption energies of Li+/BNG and Li/BNG complexes are − 47.9 kcal/mol and − 25.2 kcal/mol, respectively, that gives electrochemical cell voltage (Vcell) of 0.99 V. To increase Vcell, different halide anions such as F−, Cl−, and Br− are added to BNG. This strategy increases Vcell from 0.99 V to 3.98, 1.54, and 1.62 V for BNG/F−, BNG/Cl−, and BNG/Br− complexes, respectively. The calculated Vcell value of 3.98 V for BNG/F− is remarkable compared with previous reports in the literature. The results presented in this study may be useful for the widespread usage of BNG/F− as a promising anode material for LIBs.

Note : This data is mandatory. Please provide.



Similar content being viewed by others
References
Cheekati SL, Xing Y, Zhuang Y, Huang H (2010) Lithium storage characteristics in nano-graphene platelets. Materials challenges in alternative and renewable energy. Ceram Trans 224:117–127
Johannes MD, Swider-Lyons K, Love CT (2016) Oxygen character in the density of states as an indicator of the stability of Li-ion battery cathode materials. Solid State Ionics 286:83–89
Armand M, Tarascon JM (2008) Building better batteries. Nature 451(7179):652
Kino K, Yonemura M, Ishikawa Y, Kamiyama T (2016) Two-dimensional imaging of charge/discharge by Bragg edge analysis of electrode materials for pulsed neutron-beam transmission spectra of a Li-ion battery. Solid State Ionics 288:257–261
Prosini PP, Cento C, Carewska M, Masci A (2015) Electrochemical performance of Li-ion batteries assembled with water-processable electrodes. Solid State Ionics 274:34–39
Wang Y, Zhang C, Chen Z (2016) Model-based state-of-energy estimation of lithium-ion batteries in electric vehicles. Energy Procedia 88:998–1004
Wang Z, Wang Y, Rong Y, Li Z, Fantao L (2016) Study on the optimal charging method for lithium-ion batteries used in electric vehicles. Energy Procedia 88:1013–1017
Sun W, Liu J, Wu H, Yue X, Wang Z, Rooney D, Feng J, Sun K (2016) Facile synthesis of copper-manganese spinel anodes with high capacity and cycling performance for lithium-ion batteries. Mater Lett 182:147–150
Sato K, Noguchi M, Demachi A, Oki N, Endo M (1994) A mechanism of lithium storage in disordered carbons. Science 264(5158):556–558
Wang H, Cui LF, Yang Y, Sanchez Casalongue H, Robinson JT, Liang Y, Cui Y, Dai H (2010) Mn3O4− graphene hybrid as a high-capacity anode material for lithium ion batteries. J Am Chem Soc 132(40):13978–13980
American Association for the Advancement of Science (1997) Glasses for lithium batteries. Science 276(5317):1309
Poizot PLSG, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407(6803):496
Zhou H, Zhu S, Hibino M, Honma I, Ichihara M (2003) Lithium storage in ordered mesoporous carbon (CMK-3) with high reversible specific energy capacity and good cycling performance. Adv Mater 15(24):2107–2111
Taberna PL, Mitra S, Poizot P, Simon P, Tarascon JM (2006) High rate capabilities Fe 3 O 4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat Mater 5(7):567
C.K. Chan, H. Peng, G. Liu, K. McIlwrath, X.F. Zhang, , R.A. Huggins, and Y. Cui, High-performance lithium battery anodes using silicon nanowires. Nat Nanotechnol 3 (1) 31(2008)
Li H, Wang Z, Chen L, Huang X (2009) Research on advanced materials for Li-ion batteries. Adv Mater 21(45):4593–4607
Cabana J, Monconduit L, Larcher D, Palacin MR (2010) Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv Mater 22(35):170–192
Goodenough JB, Kim Y (2009) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603
Goriparti S, Miele E, De Angelis F, Di Fabrizio E, Zaccaria RP, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources 257:421–443
Ji L, Lin Z, Alcoutlabi M, Zhang X (2011) Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ Sci 4(8):2682–2699
Kasavajjula U, Wang C, Appleby AJ (2007) Nano-and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J Power Sources 163(2):1003–1039
Soltani A, Peyghan AA, Bagheri Z (2013) H2O2 adsorption on the BN and SiC nanotubes: a DFT study. Phys E 48:176–180
Beheshtian J, Peyghan AA, Bagheri Z (2013) Ab initio study of NH3 and H2O adsorption on pristine and Na-doped MgO nanotubes. Struct Chem 24(1):165–170
Beheshtian J, Peyghan AA, Bagheri Z (2013) Carbon nanotube functionalization with carboxylic derivatives: a DFT study. J Mol Model 19(1):391–396
Mughal MZ, Lemoine P, Lubarsky G, Maguire PD (2016) Protein adsorption on nano-patterned hydrogenated amorphous carbon model surfaces. Mater Des 97:239–248
Benjwal P, Sharma R, Kar KK (2016) Effects of surface microstructure and chemical state of featherfiber-derived multidoped carbon fibers on the adsorption of organic water pollutants. Mater Des 110:762–774
Jin P, Hou Q, Tang C, Chen Z (2015) Computational investigation on the endohedral borofullerenes M@ B 40 (M= Sc, Y, La). Theor Chem Accounts 134(2):13
Popov IA, Jian T, Lopez GV, Boldyrev AI, Wang LS (2015) Cobalt-centred boron molecular drums with the highest coordination number in the CoB 16− cluster. Nat Commun 6(1):8654
Dong H, Hou T, Lee ST, Li Y (2015) New Ti-decorated B 40 fullerene as a promising hydrogen storage material. Sci Rep 5:9952
Nejati K, Hosseinian A, Edjlali L, Vessally E (2017) The effect of structural curvature on the cell voltage of BN nanotube based Na-ion batteries. J Mol Liq 229:167–171
Yang J, Yuan Y, Hua Z (2016) Density functional theory study of interaction of graphene with hypoxanthine, xanthine, and uric acid. Mol Phys 114(14):2157–2163
Rastegar SF, Peyghan AA, Soleymanabadi H (2015) Ab initio studies of the interaction of formaldehyde with beryllium oxide nanotube. Phys E 68:22–27
Subalakshmi P, Sivashanmugam A (2017) CuO nano hexagons, an efficient energy storage material for Li-ion battery application. J Alloys Compd 690:523–531
Nayebzadeh M, Peyghan AA, Soleymanabadi H (2014) Density functional study on the adsorption and dissociation of nitroamine over the nanosized tube of MgO. Phys E 62:48–54
Xing YJ, Xi ZH, Xue ZQ, Zhang XD, Song JH, Wang RM, Xu J, Song Y, Zhang SL, Yu DP (2003) Optical properties of the ZnO nanotubes synthesized via vapor phase growth. Appl Phys Lett 83(9):1689–1691
Peyghan AA, Aslanzadeh SA, Samiei A (2014). Monatshefte Chemie-Chem Mon 145:1083–1087
Yin LW, Bando Y, Zhu YC, Li MS, Tang CC, Golberg D (2005) Single-crystalline AlN nanotubes with carbon-layer coatings on the outer and inner surfaces via a multiwalled-carbon-nanotube-template-induced route. Adv Mater 17(2):213–217
Menon M, Richter E, Mavrandonakis A, Froudakis G, Andriotis AN (2004) Structure and stability of SiC nanotubes. Phys Rev B 69(11):115322
Qie L, Chen WM, Wang ZH, Shao QG, Li X, Yuan LX, Hu XL, Zhang WX, Huang YH (2012) Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv Mater 24(15):2047–2050
Wu ZS, Ren W, Xu L, Li F, Cheng HM (2011) Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 5(7):5463–5471
Liu Y, Artyukhov VI, Liu M, Harutyunyan AR, Yakobson BI (2013) Feasibility of lithium storage on graphene and its derivatives. J Phys Chem Lett 4(10):1737–1742
Hardikar RP, Das D, Han SS, Lee KR, Singh AK (2014) Boron doped defective graphene as a potential anode material for Li-ion batteries. Phys Chem Chem Phys 16(31):16502–16508
Hosseini J, Rastgou A, Moradi R (2017) F− encapsulated B12N12 fullerene as an anode for Li-ion batteries: a theoretical study. J Mol Liq 225:913–918
Wu ZS, Winter A, Chen L, Sun Y, Turchanin A, Feng X, Müllen K (2012) Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv Mater 24(37):5130–5135
Rad AS, Zareyee D, Peyravi M, Jahanshahi M (2016) Surface study of gallium-and aluminum-doped graphenes upon adsorption of cytosine: DFT calculations. Appl Surf Sci 390:444–451
Zhang YH, Chen YB, Zhou KG, Liu CH, Zeng J, Zhang HL, Peng Y (2009) Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 20(18):185504
Zhang J, Chen J, Jiang Y, Zhou F, Wang G, Wang R (2016) Tungsten carbide encapsulated in nitrogen-doped carbon with iron/cobalt carbides electro catalyst for oxygen reduction reaction. Appl Surf Sci 389:157–164
Liu H, Liu Y, Zhu D (2011) Chemical doping of graphene. J Mater Chem 21(10):3335–3345
Hill JP, Jin W, Kosaka A, Fukushima T, Ichihara H, Shimomura T, Ito K, Hashizume T, Ishii N, Aida T (2004) Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube. Science 304(5676):1481–1483
Iyer VS, Wehmeier M, Brand JD, Keegstra MA, Müllen K (1997) From Hexa-peri-hexabenzocoronene to “Superacenes”. Angew Chem Int Ed Eng 36(15):1604–1607
Krieg M, Reicherter F, Haiss P, Ströbele M, Eichele K, Treanor MJ, Schaub R, Bettinger HF (2015) Construction of an internally B3N3-doped nanographene molecule. Angew Chem Int Ed 54(28):8284–8286
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92(1):508–517
Delley B (1996) Fast calculation of electrostatics in crystals and large molecules. J Chem Phys 100(15):6107–6110
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113(18):7756–7764
Kim E, Weck PF, Berber S, Tománek D (2008) Mechanism of fullerene hydrogenation by polyamines: Ab initio density functional calculations. Phys Rev B 78(11):113404
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Zhang H, Li WX (2009) First-principles investigation of surface and subsurface H adsorption on Ir (111). J Phys Chem C 113(51):21361–21367
Hosseinian A, Asadi Z, Edjlali L, Bekhradnia A, Vessally E (2017) NO2 sensing properties of a borazine doped nanographene: a DFT study. Comput Theor Chem 1106:36–42
Schleyer PVR, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJ (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118(26):6317–6318
Meng YS, Arroyo-de Dompablo ME (2009) First principles computational materials design for energy storage materials in lithium ion batteries. Energy Environ Sci 2(6):589–609
Gao S, Shi G, Fang H (2016) Impact of cation–π interactions on the cell voltage of carbon nanotube-based Li batteries. Nanoscale 8(3):1451–1455
Bagheri Z (2016) On the utility of C24 fullerene framework for Li-ion batteries: quantum chemical analysis. Appl Surf Sci 383:294–299
Hashemizadeh SA, Vijvieh PK, Khabir A, Najafi M (2018) Can the C32 and B16N16 nanocages be suitable anode with high performance for Li, Na and K ion batteries? Inorg Chem Commun 97:18–24
Moradi M, Bagheri Z, Bodaghi A (2017) Li interactions with the B40 fullerene and its application in Li-ion batteries: DFT studies. Phys E 89:148–154
Anaraki-Ardakani H (2017) A computational study on the application of AlN nanotubes in Li-ion batteries. Phys Lett A 381(11):1041–1046
Shakerzadeh E (2019) Functionalized olympicene (C 19 H 12) as anode material for Li-ion batteries: a DFT approach. Monatshefte für Chemie-Chemical Monthly 150(10):1745–1751
Wu X, Zhang Z, Soleymanabadi H (2020) Substituent effect on the cell voltage of nanographene based Li-ion batteries: a DFT study. Solid State Commun 306:113770
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimi, R., Solimannejad, M. The potential application of borazine (B3N3)-doped nanographene decorated with halides as anode materials for Li-ion batteries: a first-principles study. J Mol Model 26, 157 (2020). https://doi.org/10.1007/s00894-020-04418-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04418-0