Abstract
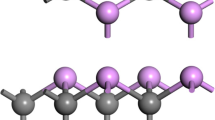
Li-ion batteries have many advantages, but these batteries suffer from safety problems, short lifetime, and a high cost. Nontoxicity, wide availability, and low cost of potassium offer the K-ion batteries (KIB) as a replacement to the Li-ion batteries. The B3LYP-gCP-D3 approach of density functional theory is applied to examine the probable application of graphyne in the anode of KIBs. It is found that a triangular hollow is the most favorable site for the K or K+ adsorption, releasing energies about 16.3 or 41.1 kcal/mol. The released energies for K and K+ have been reported to be about 16.8 and 34.2 kcal/mol for graphene sheet, respectively, which generate a cell voltage of 0.75 V. A high K storage capacity of 241 mAh/g and cell voltage of 1.08 V are predicted for graphyne. The maximum barrier energies for the displacement of K or K+ on the surface of graphyne are computed to be 2.8 (~ 3.4 for K/graphene) or 5.6 kcal/mol, representing an excellent ion mobility due to the low energy barriers. Consequently, we suggest the graphyne sheet as an anode material for the KIBs owing to its high diffusion ability, high cell voltage, and high storage capacity.



Similar content being viewed by others
References
Dahn JR, Zheng T, Liu Y, Xue J (1995) Mechanisms for lithium insertion in carbonaceous materials. Science 270:590
Johannes MD, Swider-Lyons K, Love CT (2016) Oxygen character in the density of states as an indicator of the stability of Li-ion battery cathode materials. Solid State Ionics 286:83–89
Armand M, Tarascon J-M (2008) Building better batteries. Nature 451:652–657
Kim H, Kim JC, Bianchini M, Seo DH, Rodriguez-Garcia J, Ceder G (2018) Recent progress and perspective in electrode materials for K-ion batteries. Adv Energy Mater 8:1702384
Prosini PP, Cento C, Carewska M, Masci A (2015) Electrochemical performance of Li-ion batteries assembled with water-processable electrodes. Solid State Ionics 274:34–39
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-ion batteries. Adv Funct Mater 23:947–958
(2012) “Lithium” in Mineral Commodity Summaries 2012. U.S. Geological survey, Reston, VA, p 94
(2012) “Market,” The Lithium Site. http://www.lithiumsite.com/market.html. (Accessed February 2012)
Levi E, Gofer Y, Aurbach D (2009) On the way to rechargeable mg batteries: the challenge of new cathode materials. Chem Mater 22:860–868
Barker J, Saidi MY, Swoyer JL (2003) A sodium-ion cell based on the fluorophosphate compound NaVPO4F. Electrochem Solid-State Lett 6:A1–A4
Er D, Li J, Naguib M, Gogotsi Y, Shenoy VB (2014) Ti3C2 MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries. ACS Appl Mater Interfaces 6:11173–11179
Singh N, Arthur TS, Ling C, Matsui M, Mizuno F (2013) A high energy-density tin anode for rechargeable magnesium-ion batteries. Chem Commun 49:149–151
Huie MM, Bock DC, Takeuchi ES, Marschilok AC, Takeuchi KJ (2015) Cathode materials for magnesium and magnesium-ion based batteries. Coord Chem Rev 287:15–27
Massé RC, Uchaker E, Cao G (2015) Beyond Li-ion: electrode materials for sodium-and magnesium-ion batteries. Sci China Mater 58:715–766
Besenhard JO, Winter M (2002) Advances in battery technology: rechargeable magnesium batteries and novel negative-electrode materials for lithium ion batteries. ChemPhysChem 3:155–159
Song Y-D, Wang L, Wang Q-T (2018) Computational study of the NO, SO2, and NH3 adsorptions on fragments of 3N-graphene and Al/3N graphene. J Mol Model 24:210
Moradi M, Noei M, Peyghan AA (2013) DFT studies of Si-and Al-doping effects on the acetone sensing properties of BC3 graphene. Mol Phys 111:3320–3326
Hernández Rosas JJ, Ramírez Gutiérrez RE, Escobedo-Morales A, Chigo Anota E (2011) First principles calculations of the electronic and chemical properties of graphene, graphane, and graphene oxide. J Mol Model 17:1133–1139
Yu Y-X (2013) Can all nitrogen-doped defects improve the performance of graphene anode materials for lithium-ion batteries? Phys Chem Chem Phys 15:16819–16827
Rostami Z, Soleymanabadi H (2016) N–H bond cleavage of ammonia on graphene-like B36 borophene: DFT studies. J Mol Model 22:70
Chen Y, Gao B, Zhao J-X, Cai Q-H, Fu H-G (2012) Si-doped graphene: an ideal sensor for NO- or NO2-detection and metal-free catalyst for N2O-reduction. J Mol Model 18:2043–2054
Baei MT, Peyghan AA, Bagheri Z (2013) Carbon nanocone as an ammonia sensor: DFT studies. Struct Chem 24:1099–1103
Eslami M, Peyghan AA (2015) DNA nucleobase interaction with graphene like BC3 nano-sheet based on density functional theory calculations. Thin Solid Films 589:52–56
Anota EC, Cocoletzi GH, Ramírez JFS, Hernández AB (2014) Detection of paracetamol by armchair BN nanotubes: a molecular study. Struct Chem 25:895–901
Pashangpour M, Peyghan AA (2015) Adsorption of carbon monoxide on the pristine, B-and Al-doped C3N nanosheets. J Mol Model 21:116
Beheshtian J, Noei M, Soleymanabadi H, Peyghan AA (2013) Ammonia monitoring by carbon nitride nanotubes: a density functional study. Thin Solid Films 534:650–654
Wang F, Cheng T, Zong J, Zhao M, Yang S, Song X (2019) SnO2/graphene nanocomposite coated by carbonized polyacrylic acid hydrogel as a high-performance anode for lithium-ion batteries. ChemistrySelect 4:8082–8088
Peyghan AA, Beheshtian J (2020) The influence of Stone-Wales defects in nanographene on the performance of Na-ion batteries. J Mol Graph Model 98:107578
Peyghan AA, Beheshtian J (2020) Application of hexa-peri-hexabenzocoronene nanographene and its B, N, and Bn doped forms in Na-ion batteries: a density functional theory study. Thin Solid Films 704:137979
Chen B, Chu S, Cai R, Wei S, Hu R, Zhou J (2016) First-principles simulations of lithiation–deformation behavior in silicon nanotube electrodes. Comput Mater Sci 123:44–51
Peyghan AA, Noei M (2014) A theoretical study of lithium-intercalated pristine and doped carbon nanocones. J Mex Chem Soc 58:46–51
Samadizadeh M, Rastegar SF, Peyghan AA (2015) F−, Cl−, Li+ and Na+ adsorption on AlN nanotube surface: a DFT study. Phys E 69:75–80
Lee SW, Yabuuchi N, Gallant BM, Chen S, Kim B-S, Hammond PT, Shao-Horn Y (2010) High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat Nanotechnol 5:531–537
Yan L, Yu J, Luo H (2017) Ultrafine TiO2 nanoparticles on reduced graphene oxide as anode materials for lithium ion batteries. Appl Mater Today 8:31–34
Gao B, Bower C, Lorentzen JD, Fleming L, Kleinhammes A, Tang XP, McNeil LE, Wu Y, Zhou O (2000). Chem Phys Lett 327:69–75
Haley MM (2008) Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures. Pure Appl Chem 80:519–532
Kang J, Li J, Wu F, Li S-S, Xia J-B (2011) Elastic, electronic, and optical properties of two-dimensional graphyne sheet. J Phys Chem C 115:20466–20470
Zhang Y, Pei Q, Wang C (2012) Mechanical properties of graphynes under tension: a molecular dynamics study. Appl Phys Lett 101:081909
Majidi R, Karami A (2014) Adsorption of formaldehyde on graphene and graphyne. Phys E 59:169–173
Kou J, Zhou X, Lu H, Wu F, Fan J (2014) Graphyne as the membrane for water desalination. Nanoscale 6:1865–1870
Wang A, Li L, Wang X, Bu H, Zhao M (2014) Graphyne-based carbon allotropes with tunable properties: from Dirac fermion to semiconductor. Diam Relat Mater 41:65–72
Hwang HJ, Kwon Y, Lee H (2012) Thermodynamically stable calcium-decorated graphyne as a hydrogen storage medium. J Phys Chem C 116:20220–20224
Zhang H, Zhao M, He X, Wang Z, Zhang X, Liu X (2011) High mobility and high storage capacity of lithium in sp–sp2 hybridized carbon network: the case of graphyne. J Phys Chem C 115:8845–8850
Xu Z, Lv X, Li J, Chen J, Liu Q (2016) A promising anode material for sodium-ion battery with high capacity and high diffusion ability: graphyne and graphdiyne. RSC Adv 6:25594–25600
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Wright JS, Rowley C, Chepelev L (2005) A ‘universal’B3LYP-based method for gas-phase molecular properties: bond dissociation enthalpy, ionization potential, electron and proton affinity and gas-phase acidity. Mol Phys 103:815–823
Kruse H, Goerigk L, Grimme S (2012) Why the standard B3LYP/6-31G* model chemistry should not be used in DFT calculations of molecular thermochemistry: understanding and correcting the problem. J Org Chem 77:10824–10834
Li F, Cabrera CR, Wang J, Chen Z (2016) A Cr 2 CO 2 monolayer as a promising cathode for lithium and non-lithium ion batteries: a computational exploration. RSC Adv 6:81591–81596
Chopra S (2016) Graphyne and graphdiyne: theoretical insight into ground and excited state properties. RSC Adv 6:89934–89939
Omidvar A, Mohajeri A (2015) Decorated graphyne and its boron nitride analogue as versatile nanomaterials for CO detection. Mol Phys 113:3900–3908
Lamoen D, Persson B (1998) Adsorption of potassium and oxygen on graphite: a theoretical study. J Chem Phys 108:3332–3341
Ishii A, Yamamoto M, Asano H, Fujiwara K (2008) DFT calculation for adatom adsorption on graphene sheet as a prototype of carbon nanotube functionalization. J Phys Conf Ser, 052087. IOP Publishing
Yang J, Yuan Y, Chen G (2019) First–principles study of potassium adsorption and diffusion on graphene. Mol Phys 115:1–7
Sultana I, Ramireddy T, Rahman MM, Chen Y, Glushenkov AM (2016) Tin-based composite anodes for potassium-ion batteries. Chem Commun 52:9279–9282
Chen Y, Luo W, Carter M, Zhou L, Dai J, Fu K, Lacey S, Li T, Wan J, Han X, Bao Y, Hu L (2015) Organic electrode for non-aqueous potassium-ion batteries. Nano Energy 18:205–211
Jian Z, Xing Z, Bommier C, Li Z, Ji X (2016) Hard carbon microspheres: potassium-ion anode versus sodium-ion anode. Adv Energy Mater 6:1501874
Chen C, Wang Z, Zhang B, Miao L, Cai J, Peng L, Huang Y, Jiang J, Huang Y, Zhang L (2017) Nitrogen-rich hard carbon as a highly durable anode for high-power potassium-ion batteries. Energy Storage Mater 8:161–168
Xu Y, Zhang C, Zhou M, Fu Q, Zhao C, Wu M, Lei Y (2018) Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat Commun 9:1–11
Sangavi S, Santhanamoorthi N, Vijayakumar S (2019) Density functional theory study on the adsorption of alkali metal ions with pristine and defected graphene sheet. Mol Phys 117:462–473
Meng YS, Arroyo-de Dompablo ME (2009) First principles computational materials design for energy storage materials in lithium ion batteries. Energy Environ Sci 2:589–609
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Asadi, H. High cell voltage and storage capacity of graphyne as the anode of K-ion batteries: computational studies. J Mol Model 26, 141 (2020). https://doi.org/10.1007/s00894-020-04404-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04404-6