Abstract
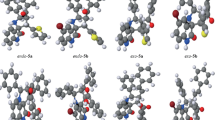
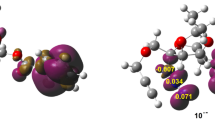
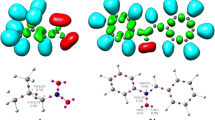
The [3+2] cycloaddition (32CA) reaction of benzonitrile oxide to α-methylene cyclopentanone and propionitrile oxide to γ-methyl-α-methylene-γ-butyrolactone, yielding regio- and stereochemically defined spiroisoxazolines, has been studied at the MPWB1K/6-311G(d,p) computational level. These processes proceed by a one-step mechanism through asynchronous transition states. Ortho regioselectivity and anti diastereofacial selectivity are predicted in complete agreement with the experimental outcomes.









Similar content being viewed by others
References
Encarnacion RD, Sandoval E, Malmstrom J, Christophersen C (2000) Calafianin, a bromotyrosine derivative from the marine sponge Aplysina gerardogreeni. J Nat Prod 63:874–875. https://doi.org/10.1021/np990489d
Bardhan S, Schmitt DC, Porco JA (2006) Total synthesis and stereochemical assignment of spiroisoxazoline natural product (+) calafianin. Org Lett 8:927–930. https://doi.org/10.1021/ol053115m
Ogamino T, Obata R, Nishiyama S (2006) Asymmetric synthesis of aerothionin, a marine dimeric spiroisoxazoline natural product, employing optically active spiroisoxazoline derivative. Tetrahedron Lett 47:727–730. https://doi.org/10.1016/j.tetlet.2005.11.097
Konig GM, Wright AD (1993) Agelorins A and B, and 11-epi-fistularin-3,three new antibacterial fistularin-3 derivatives from the tropical marine sponge Agelas oroides. Heterocycles 36:1351–1358. https://doi.org/10.3987/COM-92-6317
Perron F, Albizati KF (1989) Chemistry of spiroketals. Chem Rev 89:1617–1661. https://doi.org/10.1021/cr00097a015
Khazir J, Singh PP, Reddy M, Hyder I, Shafi S, Sawant SD, Chashoo G, Mahajan A, Alam MS, Saxena AK, Arvinda S, Gupta BD, Sampath Kumar HM (2013) Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of α-santonin. Eur J Med Chem 63:279–289. https://doi.org/10.1016/j.ejmech.2013.01.003
Blondiaux N, Moune M, Desroses M, Frita R, Flipo M, Mathys V, Soetaert K, Kiass M, Delorme V, Djaout K, Trebosc V, Kemmer C, Wintjens R, Wohlkönig A, Antoine R, Huot L, Hot D, Coscolla M, Feldmann J, Gagneux S, Locht C, Brodin P, Gitzinger M, Déprez B, Willand N, Baulard AR (2017) Reversion of antibiotic resistance in mycobacterium tuberculosis by spiroisoxazoline SMARt-420. Science 335:1206–1211. https://doi.org/10.1126/science.aag1006
Pratap S, Naaz F, Reddy S, Jha KK, Sharma K, Sahal D, Akhter M, Nayakanti D, Kumar HMS, Vandana PK, Shafi S (2019) Anti-proliferative and anti-malarial activities of spiroisoxazoline analogues of artemisinin. Arch Pharm 352:1800192. https://doi.org/10.1002/ardp.201800192
Savage GP (2010) Spiro isoxazolines via nitrile oxide 1,3-dipolar cycloaddition reactions. Curr Org Chem 14:1478–1499. https://doi.org/10.2174/138527210791616812
Zaki M, Oukhrib A, Akssira M, Berteina-Raboin S (2017) Synthesis of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of tomentosin. RSC Adv 7:6523–6529. https://doi.org/10.1039/C6RA25869G
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules, vol 4. Oxford University Press, Oxford, pp 70–86
Moss SJ, Coady CJ (1983) Potential-energy surfaces and transition state theory. J Chem Educ 60:455–461. https://doi.org/10.1021/ed060p455
Trautz M (1916) Das gesetz der reaktionsgeschwindigkeit und der gleichgewichte in gasen. Bestätigung der additivität von Cv-3/2R. Neue bestimmung der integrationskonstanten und der moleküldurchmesser. Z Anorg Allg Chem 96:1–28
Carda M, Portolés R, Murga J, Uriel S, Marco JA, Domingo LR, Zaragozá RJ, Röper H (2010) Stereoselective 1,3-dipolar cycloadditions of a chiral nitrone derived from erythrulose. An experimental and DFT theoretical study. J Org Chem 65:7000–7009. https://doi.org/10.1021/jo0009651
Jasiński R, Jasińska E, Dresler E (2017) A DFT computational study of the molecular mechanism of [3 + 2] cycloaddition reactions between nitroethene and benzonitrile N-oxides. J Mol Model 23:13. https://doi.org/10.1007/s00894-016-3185-8
Domingo LR, Ríos-Gutiérrez M, Pérez P (2018) A molecular electron density theory study of the reactivity and selectivities in [3+2] cycloaddition reactions of C,N-dialkyl nitrones with ethylene derivatives. J Org Chem 83:2182–2197. https://doi.org/10.1021/acs.joc.7b03093
Ríos-Gutiérrez M, Domingo LR (2019) Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur J Org Chem:267–282. https://doi.org/10.1002/ejoc.201800916
Domingo LR, Acharjee N (2018) [3+2] Cycloaddition reaction of C-phenyl-N-methyl nitrone to acyclic-olefin-bearing electron-donating substituent: a molecular electron density theory study. ChemistrySelect 3:8373–8380. https://doi.org/10.1002/slct.201801528
Muller G, Frischleder H, Muhlstadt M (1969) Mono- und bicyclische _- methylenketone als dipolarophile. J Prakt Chem 311:118–129. https://doi.org/10.1002/prac.19693110117
Pereira SM, Savage GP, Simpson GW, Greenwood RJ, Mackay MF (1993) Diastereoselective propionitrile oxide cycloaddition reactions with some γ-substituted α-methylene-γ-butyrolactones. Aust J Chem 46:1401–1412. https://doi.org/10.1071/CH9931401
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218. https://doi.org/10.1002/jcc.540030212
Schlegel HB (1994) Modern electronic structure theory. World Scientific Publishing, Singapore
Zhao Y, Truhlar DG (2004) Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J Phys Chem A 108:6908–6918. https://doi.org/10.1021/jp048147q
Hehre WJ, Radom L, Schleyer PVR, Pople JA (1996) Ab initio molecular orbital theory. Wiley, New York. https://doi.org/10.1002/jcc.540070314
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163. https://doi.org/10.1021/j100717a029
González C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
González C, Schlegel HB (1991) Improved algorithms for reaction path following: higher-order implicit algorithms. J Chem Phys 95:5853–5860. https://doi.org/10.1063/1.461606
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Simkin BY, Sheikhet II (1995) Quantum chemical and statistical theory of solutions: a computational approach. Ellis Horwood, London
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335. https://doi.org/10.1016/0009-2614(96)00349-1
Cances E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041. https://doi.org/10.1063/1.474659
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748 (22 pages). https://doi.org/10.3390/molecules21060748
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org. Biomol Chem 9:7168–7175. https://doi.org/10.1039/C1OB05856H
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494. https://doi.org/10.1039/C2RA22886F
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision D.01. Gaussian, Inc., Wallingford CT
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403. https://doi.org/10.1063/1.458517
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371:686–686. https://doi.org/10.1038/371683a0
Yepes D, Murray JS, Pérez P, Domingo LR, Politzer P, Jaque P (2014) Complementarity of reaction force and electron localization function analyses of asynchronicity in bond formation in Diels–Alder reactions. Phys Chem Chem Phys 16:6726–6734. https://doi.org/10.1039/C3CP54766C
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon Press, Oxford
Aurell MJ, Domingo LR, Pérez P, Contreras R (2004) A theoretical study on the regioselectivity of 1,3-dipolar cycloadditions using DFT-based reactivity indexes. Tetrahedron 60:11503–11509. https://doi.org/10.1016/j.tet.2004.09.057
Benchouk W, Mekelleche SM, Silvi B, Aurell MJ, Domingo LR (2011) Understanding the kinetic solvent effects on the 1,3-dipolar cycloaddition of benzonitrile N-oxide: a DFT study. J Phys Org Chem 24:611–618. https://doi.org/10.1002/poc.1858
Ndassa IM, Adjieufack AI, Ketcha JM, Berski S, Ríos-Gutiérrez M, Domingo LR (2017) Understanding the reactivity and regioselectivity of [3+2] cycloaddition reactions between substituted nitrile oxides and methyl acrylate. A molecular electron density theory study. Int J Quantum Chem 117:e25451. https://doi.org/10.1002/qua.25451
Acknowledgments
The author is thankful to Professor Manas Banerjee, Retired Professor, at The University of Burdwan, India, for his kind cooperation. The author also acknowledges the help and support of Professor Luis R Domingo, Professor, at the University of Valencia, Spain, for important clarifications in several studies related to the concept of 32CA reactions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Tables with the MPWB1K/6-311G(d,p) electronic energies, enthalpies and Gibbs free energies of the stationary points involved in the 32CA reactions of nitrile oxides 1 and 4 with MCP 2 and MBL 5, in gas phase, THF and benzene. ELF topological analysis at the reactants and TSs and calculations of QTAIM parameters. (DOCX 1124 kb).
Rights and permissions
About this article
Cite this article
Acharjee, N. Theoretical analysis of the regio- and stereoselective synthesis of spiroisoxazolines. J Mol Model 26, 117 (2020). https://doi.org/10.1007/s00894-020-04372-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04372-x