Abstract
The α-H acidity is an important chemical property of ketones that has attracted much research interest. Theoretical prediction of pKa for ketone α-H is significant. In this work, we theoretically studied the nuclear shielding of various α-Hs in a set of ketones and that of the corresponding enolic hydroxyl Hs in tautomeric enol forms. It has been demonstrated through linear regression analyses that the pKa values of these ketones correlate with both sets of the calculated nuclear shielding values. The correlation coefficient R2 of the linear correlation relationship is 0.90. The present work has provided a new approach to computationally evaluating the acidity of α-Hs in ketones, enabling us to semi-empirically predict the ketone α-H acidity from the calculated nuclear shielding values.

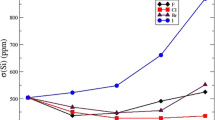
Experimental pKa values in DMSO vs predicted pKa values calculated from 1H nuclear shielding for the hydroxyl hydrogens in the enol forms and for the α-Hs in the keto forms. The surrounding solvent effects were modelled by keto/enol-DMSO clusters and SMD solvent models





Similar content being viewed by others
References
Guthrie JP, Cossar J, Klym A (1982) The pKa of acetone: a kinetic method for determining the pKas of ketones in aqueous solution. J Am Chem Soc 104:895–896
Albro PW, Parker CA, Abusteit EO, Mester TC, Hass JR, Sheldon YS, Corbin FT (1984) Determination of the pKA values of metribuzin and three of its metabolites: a comparison of spectrophotometric and potentiometric methods. J Agric Food Chem 32:212–217
Garrido G, Koort E, Ràfols C, Bosch E, Rodima T, Leito I, Rosés M (2006) Acid−base equilibria in nonpolar media. Absolute pKa scale of bases in tetrahydrofuran. J Organomet Chem 71:9062–9067
Bordwell FG (1988) Equilibrium acidities in dimethyl sulfoxide solution. Acc Chem Res 21:456–463
Bors DA, Kaufman MJ, Streitwieser A (1985) Carbon acidity. 67. The indicator scale of cesium ion pairs in tetrahydrofuran. J Am Chem Soc 107:6975–6982
Kolling OW (1974) Empirical acidity functions for strongly basic solutions in the binary solvent: water-dimethyl sulfoxide 76:193–193
Cox RA, Yates K (2011) Acidity functions: an update 61:2225–2243
Nakamura S, Hirao H, Ohwada T (2004) Rationale for the acidity of Meldrum’s acid. consistent relation of C−H acidities to the properties of localized reactive orbital. J Org Chem 69:4309–4316
Khursan SL, Ovchinnikov MY (2014) The pK(a) theoretical estimation of C-H, N-H, O-H and S-H acids in dimethylsulfoxide solution. J Phys Org Chem 27:926–934
Lu J, Lu T, Zhao X, Chen X, Zhan C-G (2018) Correlations between the 1H NMR chemical shieldings and the pKa values of organic acids and amines. J Mol Model 24:146
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37:785–789
Becke A (1993) D., Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041
Cossi M, Barone V (1998) Analytical second derivatives of the free energy in solution by polarizable continuum models. J Chem Phys 109:6246–6254
Mennucci B, Cammi R, Tomasi J (1998) Excited states and solvatochromic shifts within a nonequilibrium solvation approach: a new formulation of the integral equation formalism method at the self-consistent field, configuration interaction, and multiconfiguration self-consistent field level. J Chem Phys 109:2798–2807
Szelejewska-Woźniakowska A, Chilmonczyk Z, Leś A, Cybulski J, Wawer I (1999) 13-C cross-polarization magic angle spinning NMR and gauge-independent atomic orbital, coupled Hartree–Fock calculations of buspirone analogues: Part 2. Hydrochlorides and perchlorates of 1-arylpiperazine-4-alkylimides. Solid State Nucl Magn Reson 14:59–65
Frisch MJ, Schlegel GWTHB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian(R) 09 program. Gaussian, Inc, Wallingford
Funding
The work was supported by the National Natural Science Foundation of China (grant number 21273089), the Fundamental Research Funds for the South-Central University for Nationalities (CZW17004), the Open Project Fund of the Key Laboratory of the Pesticides and Chemical Biology of Central China Normal University (grant number 2018-A01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Optimized geometries and predicted 1H nuclear shieldings for the solute-DMSO clusters. Experimental pKa values for the ketones in this study. (DOCX 5164 kb)
ESM 1
(DOCX 5164 kb)
Rights and permissions
About this article
Cite this article
Xing, S., Lu, J., Zhao, X. et al. Correlation between the pKa and nuclear shielding of α-hydrogen of ketones. J Mol Model 25, 354 (2019). https://doi.org/10.1007/s00894-019-4244-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4244-8