Abstract
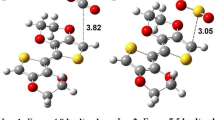
In this study, we carried out with the DTP (dithieno(3,2-b:2,3-d) pyrrole) molecule which is a member of the conductive polymer class that is one of the topics that have been studied prevalently in recent times, and the density functional theory’s (DFT) calculation methods were used to shed light on the sensor mechanisms of its interactions with SOx (SO2 and SO3) sulfur oxides. The changes in the geometric and electronic characteristics of a sensor mechanism designed with the DTP molecule when it encountered sulfur oxides were examined. With these changes, the usability of DTP as a sensor material was proven. DTP sensor applications using this method were not investigated in previous studies. The B3LYP 6–31 G(d) levels of DFT were used in the calculations. In the calculations, during the interaction between analyte (SO2-SO3) and the conductive polymer DTP (dithieno(3,2-b:2,3-d) pyrrole), especially, the changes in its geometric and electronic structures were observed. With these changes that were observed in the geometric structure, as a result of the interaction between the conductive polymer and gas molecules, the resistance on the polymer’s main chain decreased, and conductivity increased. Calculations on the bandgap on HOMO-LUMO energy levels were observed to decrease. Thus, the structural conductivity of the molecule increased. Additionally, the experiments showed that, as a result of interaction with gas molecules, the bandgap in the ionization potential, electron affinity, and HOMO-LUMO energy levels varied. These variations showed the detection mechanisms for sulfur oxides by the DTP (dithieno(3,2-b:2,3-d) pyrrole) molecule that may be used to design sensors.




Similar content being viewed by others
References
Iwasawa S, Kikuchi Y, Nishiwaki Y et al (2009) Effects of SO2 on respiratory system of adult Miyakejima resident 2 years after returning to the island. J. Occup. Health 51:38–47
Du SX, JinHF BDF, ZhaoX TCS, Du JB (2008) Endogenously generated sulfur dioxide and its vasorelaxant effect in rats. Acta Pharmacol Sin 29:923–930
Rad AS, Valipour P, Gholizade A, Mousavinezhad SE (2015) Interaction of SO2 and SO3 on terthiophene (as a model of polythiophenegassensor): DFT calculations. ChemPhysLett 639(29–35):306
Ali SR, Nazanin N, Maryam J, Dorsa SS, Elahe G (2015) Ab-initiostudy of interaction ofsomeatmosphericgases (SO2, NH3, H2O, CO, CH4 and CO2) withpolypyrrole (3PPy) gassensor: DFT calculations. SensorsActuators. B: Chemical 220:641–651
Ullah H, Ayub K, Ullah Z (2013) Theoretical insight of polypyrrole ammonia gas sensor. Synthetic Metals 172:14–20
Sajid H, Mahmood T, Ayub K (2018) High sensitivity of polypyrrole sensor for uricacid over urea, acetamide and sulfonamide: a density functional theory study. Synth. Met. 235:49–60
Shokuhi Rad A, Zardoost MR, Abedini E (2015) First-principles study of terpyrrole as a potential hydrogencyanide sensor: DFT calculations. J. Mol. Model. 21:273
Bibi S, Ullah H, Ahmad SM (2015) Molecular and electronic structure elucidation of polypyrrole gas sensors. J PhysChem C 119:15994–16003
Sajid H, Mahmood T, Ayub K (2017) An accurate comparative theoretical study of the interaction of furan, pyrrole, and thiophene with various gaseous analytes. J Mol Model 23:1–18
Liu SS, Bian LJ, Luan F (2012) Theoretical study on polyaniline gas sensors: examinations of response mechanism for alcohol. Synth. Met. 162:862–867
Bredas JL, Street GB (1985) Polarons, bipolarons, andsolitons in conductingpolymers. AccChemRes 18:309–315
Hacer A, Sevınc K, Huseyın Bekır Y, Sıbel AO (2016) Electrochemical glucose biosensing via new generation DTP type conducting polymers/gold nanoparticles/glucose oxidase modified electrodes. Journal of Elecanalytical Chemistry 770:90–97. https://doi.org/10.1016/j.jelechem.2016.03.034
Bai H, Shi G, Bai H, Shi G (2007) Gas Sensors Based on Conducting Polymers. Sensors 7:267–307
Gospodinova N, Mokreva P, Terlemezyan L (1993) Chemical oxidative polymerization of aniline in aqueous medium without added acids. Polymer. 34:336
Sahiner N, Demirci S (2016) Conducting semi-inter penetrating polymeric composites via the preparation of poly(aniline), poly(thiophene), and poly(pyrrole) polymers within superporous poly(acrylicacid) cryogels. React. Funct. Polym. 340(105):60–65
Zade SS, Bendikov M (2009) In: Perepichka IF, Perepichka DF (eds) Theoretical studies on thiophene-containing compounds. John Wiley&Sons, Ltd., New York ISBN: 978-0-470-05732-2
Puodziukynaite E, Wang H-W, Lawrence J et al (2014) Azulene methacrylate polymers: synthesis, electronic properties, and solar cell fabrication. J AmChemSoc 136:11043–11049
Bozkurt A, Akbulut U, Toppare L (1996) Conducting polymer composites of polypyrrole and polyindene. Synth. Met. 82:41
Ullah H, Shah A-HA, Bilal S, Ayub K (2014) Doping and dedoping processes of polypyrrole: DFT study with hybrid functionals. J. Phys. Chem. C 118:17819–17830
Kassim A, Basar ZB, Mahmud HNME (2002) Effects of preparation temperature on the conductivity of polypyrrole conducting polymer. J ChemSci 114:155–162
Alex LG, Jordi C, Oscar B, JoãoSinézio CC, Elaine A, Carlos A (2011) Electronic properties of poly(thiophene-3-methyl acetate). J PolymRes 18:1509–1517
Zade SS, Bendikov M (2006) From oligomers to polymer: convergence in the HOMO-LUMO gaps of conjugated oligomers. Org Lett 8:5243–5246
Yoon H (2013) Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 3:524–549
Hutchison GR, Zhao Y-J, Delley B et al (2003) Electronic structure of conducting polymers: limitations of oligomer extra polation approximation sand effects of heteroatoms. PhysRev B 68:035204
Alemán C, Estrany F, Armelin E (2007) A theoretical study on the interaction between N-methylpyrroleand 3,4-ethylenedioxythiophene units in copolymermolecules. Polymer (Guildf) 48:6162–6169
Rikukawa M, Sanui K (2000) Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. ProgPolymSci 25:1463–1502
Gustafsson G, Cao Y, Treacy GM, Klavetter F, Colaneri N, Heeger AJ (1992) Flexible light-emitting diodes made from soluble conducting polymers. Nature 357:477–479
Watt A, Blake D, Warner JH, Thomsen EA, Tavenner EL Dunlop HR, Meredith P (2005). J. Phys. D. Appl. Phys. 38:2006–2012
Masoumeh M, Morteza MK, Mina G (2019) Exploringtheeffect of phosphorusdoping on theutility of g-C3N4 as an electrodematerial in Na- ionbatteriesusing DFT method. Journal of MolecularModeling 375. https://doi.org/10.1007/s00894-019-4109-1
Frisch MJEA, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman, JR.(2000) Gaussian 09, Revision a. 02, Inc.,Wallingford, CT
Foresman JB, Frish E (1996) Exploring chemistry with electronic structure methods2nd edn. Gaussian, Inc., Pittsburgh PA USA, p 253
Mulliken RS (1934) A new electron affinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 2:782–793
Koch W, Holthausen MC (2001) A chemist’s guide to density functional theory. Wiley-VCH VerlagGmbH
Hasnain S, Tarıq M, Mian HRM, Khurshid A (2019) Comparative investigation of sensor application of polypyrrole for gaseous analytes. Journal of physical organic chemistry. https://doi.org/10.1002/poc.3960
Pandule SS, Shisodia SU, Pawar RP, Chabukswar VV (2017) Synthesis, properties, and ammonia gas sensing applications of poly-[1-(4-nitronaphthalen-1-yl)-2,5- di(thiophen-2-yl)-1 H -pyrrole]. Polym Plast Technol Eng. 56:268–275
Xiaohua X, Wei S, Yangwu F, Ming L (2010) DFT study of conductive properties of three polymers formed by bicyclic furans. Journal Molecular Simulation 36:836–398 846
Ali SR, PeimanV AG, Seyed EM (2015) Interaction of SO2 and SO3 on terthiophene (as a model of polythiophene gas sensor): DFT calculations. Chemical Physics Letters 639:29–35
Sajid H, Mahmood T, Ayub K (2018) High sensitivity of polypyrrole sensor for uric acid over urea, acetamide and sulfonamide: a density functional theory study. Synth Met 235:49–60
Shokuhi RA, GhasemiAteni S, Tayebi H (2016) First-principles DFT study of SO2 and SO3 adsorption on 2PANI: a model for polyaniline response. J Sulfur Chem.:1–10
Shokuhi RA, Esfahanian M, Ganjian E, Tayebi H (2016) Ab-initio study of physisorption of hydrogen cyanide on 2PANI: a model for polyaniline gas sensor. Z. Phys. Chem. 230:1487–1498
Rasmussen SC, Evenson SJ, McCausland CB (2015) Fluorescent thiophene-based materials and their Outlook for Emissive Applications. Chem. Commun. 51:4528–4543
Funding
This study was financially supported by the Karamanoglu Mehmetbey University Research Foundation (grant number 14-M-17; 02-YL-18).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• We theoretically studied the interactions of SO2 and SO3 with conductive polymer through DFT calculations.
• Geometric optimization orbital analysis and UV-VIS results were calculated theoretically.
• The results show dithieno(3,2-b:2,3-d) pyrrole (DTP) that is a member of the conductive polymer class interacts strongly with SO2 and SO3 gas molecules and can be used in gas sensors.
Rights and permissions
About this article
Cite this article
AZAK, H., GORGUL, R., TEKIN, B. et al. Calculation of conductive polymer-based SO2 and SO3 gas sensor mechanisms by using the DFT method. J Mol Model 25, 367 (2019). https://doi.org/10.1007/s00894-019-4219-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4219-9