Abstract
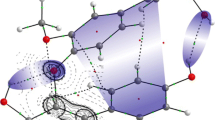
Lignin is one of the most abundant natural materials around the world, accounting for about a quarter of the woody tissue. In general, it is well known that these highly branched aromatic bio-polymers are formed from the polymerization of p-coumaryl, coniferyl, and sinapyl alcohols; however, the connection between these structures are still not known in detail. In this work, we have employed electronic structure calculations to investigate local reactivities and details regarding the connectivity between the basic structures of lignin (unmodified mono and dilignols as well as dehydrogenated monolignols). Condensed-to-atoms Fukui indexes, local softness and hard and soft acids and bases principle were employed in the analyses. The results allow identifying reactive sites on the lignin subunits and access details on the synthesis and degradation of this bio-material. In particular, it is possible to identify a strong influence of the dehydrogenation and monomer dimerization on the monolignols reactivities, which activate the O–C4 and C5 positions.

The local reactivities of lignin subunits were evaluated via DFT calculations.





Similar content being viewed by others
References
Boerjan W, Ralph J, Baucher M (2003) . Ann Rev Plant Biol 54(1):519. https://doi.org/10.1146/annurev.arplant.54.031902.134938
Freudenberg K (1966) .. In: Lignin structure and reactions, advances in chemistry, vol 59. American Chemical Society, pp 1–21
Higuchi T (2006) . J Wood Sci 52(1):2. https://doi.org/10.1007/s10086-005-0790-z
Sjöström E (1993) Wood chemistry: fundamentals and applications. Gulf Professional Publishing
Chen H (2014) .. In: Biotechnology of lignocellulose. Springer, Netherlands, pp 25–71
Pettersen R (1984) . In: Rowell R (ed) The chemistry of solid wood, vol 207. American Chemical Society, Washington, pp 57–126. https://doi.org/10.1021/ba-1984-0207.ch002
Rowell RM, Pettersen R, Tshabalala MA (2013) . In: Rowell R (ed) Handbook of wood chemistry and wood composites, Chapter 3. 2nd edn. CRC Press, Boca Raton, pp 33–72
Elder T, Fort RC Jr (2010) . In: Heitner C, Dimmel D, Schmidt JA (eds) Lignin and lignans: advances in chemistry. Taylor & Francis, Boca Raton, pp 321–348
Durbeej B, Wang YN, Eriksson L (2003) . In: Goos G, Hartmanis J, van Leeuwen J, Palma JMLM, Sousa AA, Dongarra J, Hernández V (eds) High performance computing for computational science — VECPAR 2002, vol 2565. Springer, Berlin, pp 137–165
Durbeej B, Eriksson L (2003) . Holzforschung 57(1):59. https://doi.org/10.1515/HF.2003.009
Durbeej B, Eriksson L (2003) . Holzforschung 57(2):150. https://doi.org/10.1515/HF.2003.024
Sangha AK, Petridis L, Smith JC, Ziebell A, Parks JM (2012) . Environ progress sustain energy 31(1):47. https://doi.org/10.1002/ep.10628
Martinez C, Rivera JL, Herrera R, Rico JL, Flores N, Rutiaga JG, López P (2008) . J Mol Model 14(2):77. https://doi.org/10.1007/s00894-007-0253-0
Martínez C, Sedano M, Mendoza J, Herrera R, Rutiaga JG, Lopez P (2009) . J Mol Graph Modell 28(2):196. https://doi.org/10.1016/j.jmgm.2009.07.002
Shigematsu M, Kobayashi T, Taguchi H, Tanahashi M (2006) . J Wood Sci 52(2):128. https://doi.org/10.1007/s10086-005-0737-4
Higuchi T (1985) .. In: Biosynthesis and biodegradation of wood components. Elsevier, pp 141–160. https://doi.org/10.1016/B978-0-12-347880-1.50011-8
Batagin-Neto A, Oliveira EF, Graeff C, Lavarda F (2013) . Mol Simul 39(4):309. https://doi.org/10.1080/08927022.2012.724174
Batagin-Neto A, Bronze-Uhle E, Graeff CFO (2015) . Phys Chem Chem Phys 17(11):7264. https://doi.org/10.1039/C4CP05256K
Schaftenaar G, Noordik JH (2000) . J Comput-Aided Mol Des 14(2):123. https://doi.org/10.1023/A:1008193805436
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) . J Comput Chem 25(9):1157. https://doi.org/10.1002/jcc.20035
Allouche AR (2011) . J Comput Chem 32(1):174. https://doi.org/10.1002/jcc.21600
Stewart JJP (2007) . J Mol Model 13(12):1173. https://doi.org/10.1007/s00894-007-0233-4
Stewart JJP (1990) . J Comput-Aided Mol Des 4(1):1. https://doi.org/10.1007/BF00128336
Gans JD, Shalloway D (2001) . J Mol Graph Modell 19(6):557. https://doi.org/10.1016/S1093-3263(01)00090-0
Becke AD (1993) ., vol 98. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) . Phys Rev B 37(2):785. https://doi.org/10.1103/PhysRevB.37.785
Vosko SH, Wilk L, Nusair M (1980) . Can J Phys 58(8):1200. https://doi.org/10.1139/p80-159
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) . J Phys Chem 98(45):11623. https://doi.org/10.1021/j100096a001
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H (2009) Gaussian 09
Klamt A, Schüürmann G (1993) . J Chem Soc Perkin Trans 2(5):799. https://doi.org/10.1039/P29930000799
Jensen F (2006) Introduction to computational chemistry, 2nd edn. Wiley, New York
Yang W, Mortier WJ (1986) . J Amer Chem Soc 108(19):5708. https://doi.org/10.1021/ja00279a008
Zielinski F, Tognetti V, Joubert L (2012) . Chem Phys Lett 527:67. https://doi.org/10.1016/j.cplett.2012.01.011
Chermette H (1999) . J Comput Chem 20(1):129
Geerlings P, De Proft F, Langenaeker W (2003) . Chem Rev 103(5):1793. https://doi.org/10.1021/cr990029p
Domingo L, Ríos-Gutiérrez M, Pérez P (2016) . Molecules 21(6):748. https://doi.org/10.3390/molecules21060748
Lewars EG (2010) Computational chemistry: introduction to the theory and applications of molecular and quantum mechanics, 2nd edn. Springer, Berlin
Batagin-Neto A, Bronze-Uhle E, Vismara M, Assis A, Castro F, Geiger T, Lavarda F, Graeff C (2013) . Current Phys Chem 3(4):431. https://doi.org/10.2174/18779468113036660026
Bronze-Uhle E, Batagin-Neto A, Lavarda F, Graeff CFO (2011) . J Appl Phys 110(7):073510. https://doi.org/10.1063/1.3644946
Cesarino I, Simões RP, Lavarda F, Batagin-Neto A (2016) . Electrochim Acta 192:8. https://doi.org/10.1016/j.electacta.2016.01.178
Martins LM, de Faria Vieira S, Baldacim GB, Bregadiolli BA, Caraschi JC, Batagin-Neto A, da Silva-Filho LC (2018) . Dye Pigment 148:81. https://doi.org/10.1016/j.dyepig.2017.08.056
Mandú LO, Batagin-Neto A (2018) . J Mol Model 24(7):157. https://doi.org/10.1007/s00894-018-3660-5
do Amaral Rodrigues J, de Araújo AR, Pitombeira NA, Plácido A, de Almeida MP, Veras LMC, Delerue-Matos C, Lima FCDA, Neto AB, de Paula RCM, Feitosa JPA, Eaton P, Leite JRSA, da Silva DA (2019) . Int J Biol Macromol 128:965. https://doi.org/10.1016/j.ijbiomac.2019.01.206
De Proft F, Martin JM, Geerlings P (1996) . Chem Phys Lett 256(4-5):400. https://doi.org/10.1016/0009-2614(96)00469-1
Thanikaivelan P, Padmanabhan J, Subramanian V, Ramasami T (2002) . Theor Chem Accounts: Theory, Comput Model (Theor Chim Acta) 107(6):326. https://doi.org/10.1007/s00214-002-0352-z
Roy RK, Pal S, Hirao K (1999) . J Chem Phys 110(17):8236. https://doi.org/10.1063/1.478792
De Proft F, Van Alsenoy C, Peeters A, Langenaeker W, Geerlings P (2002) . J Comput Chem 23 (12):1198. https://doi.org/10.1002/jcc.10067
Fleming I (2007) Frontier orbitals and organic chemical reactions, Reprint edn. Wiley, London
Eider T, McKee M, Worley S (1988) . Holzforschung 42(4):233. https://doi.org/10.1515/hfsg.1988.42.4.233
Elder T, Worley S (1985) . Holzforschung 39(3):173. https://doi.org/10.1515/hfsg.1985.39.3.173
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) . Plant Physiology 153(3):895. https://doi.org/10.1104/pp.110.155119
Dimmel D (2010) . In: Heitner C, Dimmel D, Schmidt JA (eds) Lignin and lignans: advances in chemistry. Taylor & Francis, Boca Raton, pp 1–10
Funding
The authors thank the Brazilian National Council for Scientific and Technological Development (CNPq) [grant numbers 448310/2014-7 and 420449/2018-3] and the Pro-Rectory of Research (PROPe) of the São Paulo State University (UNESP) for the financial support and student scholarship. This research was also supported by resources supplied by the Center for Scientific Computing (NCC/GridUNESP) of the São Paulo State University (UNESP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maia, R.A., Ventorim, G. & Batagin-Neto, A. Reactivity of lignin subunits: the influence of dehydrogenation and formation of dimeric structures. J Mol Model 25, 228 (2019). https://doi.org/10.1007/s00894-019-4130-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4130-4