Abstract
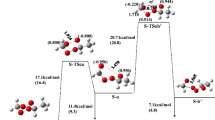
Organic peroxides are interesting compounds with a broad range of properties from antimalarial and antimicrobial activities to explosive character. In this work the gas-phase thermolysis reaction mechanism of the 3,6-dimethyl-1,2,4,5-tetroxane (DMT) is studied by DFT calculations, considering axial–axial, axial–equatorial, and equatorial–equatorial position isomers. The critical points of the singlet (S) and triplet (T) potential energy surfaces (PES) are calculated. Three mechanisms are considered: i) S-concerted, ii) S-stepwise, and iii) T-stepwise. The first intermediate of the reaction through S-stepwise-PES is a diradical open structure, o, yielding, as products, two molecules of acetaldehyde and one of O2 in the S state. The S-stepwise-mechanism gives exothermic reaction energies (Er) in the three position isomers. The S-concerted mechanism yields very high activation energies (Ea) in comparison with those of the S-stepwise mechanism. In the T-stepwise mechanism, a triplet open structure (T-o) is first considered, yielding an Er 12 kcal mol–1 more exothermic than that of the S-mechanisms. The S-o and T-o are similar in structure and energies; therefore, a crossing from the S- to T-PES is produced at the o intermediate as a consequence of a spin–orbit coupling. The highest Ea is the first step after o intermediate, and thus it is considered the rate limiting step. Therefore, the Er at the T-PES is more in agreement with the Er of the exothermic experimental diperoxide products. Ea, Er, and O···O distances are studied as a function of the number of methyl groups and the position isomerization.













Similar content being viewed by others
References
Cerna J, Morales G, Eyler G, Cañizo A (2002) Bulk polymerization of styrene catalyzed by biand tri-functional cyclic initiators. J Appl Polym Sci 83:1–11
Nesprías K, Cañizo A, Eyler N, Mateo C (2004) Oxidación de alcoholes utilizando peróxidos orgánicos cíclicos polifuncionales. Afinidad 61:471–475
Angelis YS, Hatzakis NS, Smonou I, Orfanopoulos M (2001) Oxidation of benzyl alcohols by dimethyldioxirane. The question of concerted versus stepwise mechanism probed by kinetic isotope effects. Tetrahedron Lett 42:3753–3756
Vennerstrom JL, Ager AL, Andersen SL, Grace JM, Wongpanich V, Angerhofer CK (2000) Assessment of the antimalarial potential of tetraoxane. Am J Trop Med Hyg 62(5):573–578
Sanderson JR, Story PR (1974) Macrocyclic synthesis. The thermal decomposition of dicyclohexylidene diperoxide and tricyclohexylidene triperoxide. J Org Chem 39:3463–3469
Milas NA, Golubovic A (1959) Studies in organic peroxides. XXVI. Organic peroxides derived from acetone and hydrogen peroxide. J Am Chem Soc 81(24):6461–6462
Oxley JC, Smith JL, Chen H (2002) Decomposition of a multiperoxidic compound: triacetone triperoxide (TATP). Propellants Explos Pyrotech 27:209–216
Leiva LCA, Jorge NL, Gómez Vara ME (1999) Síntesis Modificada del peróxido dimérico de acetaldehído (DMT) Reuniones Científicas y Tecnológicas de Ciencia y Técnica -UNNE. E-037.pdf. http://www.unne.edu.ar/unnevieja/Web/cyt/cyt/cyt2000.htm
Leiva LCA, Jorge NL, Romero JM, Cafferata LFR, Gómez Vara ME, Castro EA (2008) Decomposition of the acetone cyclic diperoxide in octanol solution. J Argent Chem Soc 96(1–2):110–122
Leiva LCA, Jorge NL, Romero JM, Cafferata LFR, Gómez Vara ME, Castro EA (2010) Thermal decomposition reaction of 3,3,6,6- tetramethyl- 1,2,4,5- tetroxane in 2-methoxy-ethanol solution. Chem Heterocycl Compd 45(12):1455–1459
Pila AN, Profeta MI, Romero JM, Jorge NL, Castro EA (2012) Kinetics and mechanism of the thermal decomposition reaction of 3, 6-diphenyl-1,2,3,5-tetroxane in solution. Int J Chem Model 4(4):5–10
Profeta MI, Romero JM, Leiva LCA, Jorge NL, Gómez Vara ME, Castro EA (2011) Solvent effect of oxygen in the thermolisys decomposition of the acetone diperoxide. Meth Appl Chemoinf Chem Eng 96(1–2):110–122
Reguera MB, Frette SG, Romero JM, Jorge NL, Castro EA (2012) Synthesis and thermical decomposition reaction of 3,6-dibutanoic-1, 2,4,5-tetroxane in solution. Bentham Sci Newsl 4(1):1–4
Jorge NL, Romero JM, Grand A, Hernández-Laguna A (2012) Gas-phase thermolysis reaction of formaldehyde diperoxide. Kinetic study and theoretical mechanisms. Chem Phys 39:37–45
Profeta MI, Romero JM, Jorge NL, Grand A, Hernández-Laguna A (2014) Theoretical study of the gas-phase thermolysis of 3-methyl-1,2,4,5-tetroxane. J Mol Model 20:2224
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Slater JC (1974) Quantum theory of molecules and solids, the selfconsistent field for molecules and solids, vol 4. McGraw-Hill, New York
Lee C, Yang LW, Par RG (1988) Development of the Colle–Salvetti correlation energy formula into a functional of the electron density. Phys Rev B 37:785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2013) Gaussian 09, revision E.01. Gaussian Inc., Wallingford
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218
Baker J (1986) An algorithm for the location of transition states. J Comput Chem 7:385–395
Baker J (1987) An algorithm for geometry optimization without analytical gradients. J Comput Chem 8(5):563–574
Flúkiger P, Lúthi HP, Portmann S, Weber J (2000) Gaussview 5.0. Swiss Center for Scientific Computing, Manno
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in massweighted internal coordinates. J Phys Chem 94:5523–5527
Abegg PW, Ha TK (1974) Ab initio calculation of spin-orbit coupling constant from Gaussian lobe SCF molecular wavefunctions. Mol Phys 27:763–767
Abegg PW (1975) Ab initio calculation of spin-orbit-coupling constants for Gaussian lobe and Gaussian-type wave-functions. Mol Phys 30:579–596
Koseki S, Gordon MS, Schmidt MW, Matsunaga N (1995) Maingroup effective nuclear charges for spin-orbit calculations. J Phys Chem 99:12764–12772
Koseki SS, Schmidt MW, Gordon MS (1998) Effective nuclear charges for the first- through third-row transition metal elements in spin-orbit calculations. J Phys Chem 102:10430–10435
Eade RHE, Robb MA (1981) Direct minimization in MC SCF theory: the quasi-Newton method. Chem Phys Lett 83:362–368
Siegbahn EM (1984) A new direct CI method for large CI expansions in a small orbital space. Chem Phys Lett 109:417–423
Bernardi F, Olivucci M, Robb MA (1990) Mechanism of ground-state-forbidden photochemical pericyclic reactions: evidence for real conical intersections. J Am Chem Soc 112:1737–1744
Klene M, Robb MA, Frisch MJ, Celani P (2000) Parallel implementation of the CI-vector evaluation in full CI/CAS-SCF. J Chem Phys 113:5653–5665
Lefller JE (1953) Parameters of the description of the transition state. Science 117:340–341
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338
Acknowledgments
The authors thank the “Centro de Supercomputación de Galicia” (CESGA), and “Centro de Servicios de Informática y Redes de Comunicaciones (CSIRC), Universidad de Granada” (Spain) for providing the computing time. The authors are thankful to “Secretaría General de Ciencia y Técnica de la UNNE (Argentina)”. This work was supported by Spanish MCINN and European FEDER grants FIS2016-77692-C2-2P, PCIN-2017-098 and by the regional agency “Junta de Andalucía” for the RNM-264, -363 and -1897 PAI-grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Electronic supplementary material
ESM 1
(DOCX 41.0 kb)
Rights and permissions
About this article
Cite this article
Bordón, A.G., Pila, A.N., Profeta, M.I. et al. Theoretical study of the gas-phase thermolysis reaction of 3,6-dimethyl-1,2,4,5-tetroxane. Methyl and axial-equatorial substitution effects. J Mol Model 25, 217 (2019). https://doi.org/10.1007/s00894-019-4092-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4092-6