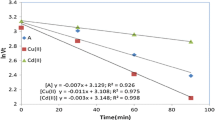
Abstract
The reaction of the Δ4-alkenols with PhSeX can follow three possible reaction pathways: two pathways lead to the formation of two regioisomeric cyclic ether products through the process of intramolecular cyclization, while the third represents the addition of the reagent to the double bond of an alkenol. As there are relatively few literature data on the kinetics of these reactions, we have chosen 6-methyl-hept-5-en-2-ol as a substrate of interest in order to obtain valuable results that will enable better understanding of the mechanism of phenylselenoetherification reactions. 6-Methyl-hept-5-en-2-ol is a particularly interesting model-substrate due to its substitution pattern of functional groups involved in the cyclization process. In this research, through synthetic and kinetic studies, we aimed to resolve key questions concerning the influence on kinetics, chemo- and regioselectivity of the reagent’s counter ion, steric hindrances in substrate functional groups and the presence of additives.





Similar content being viewed by others
References
Kumar Y, Green R, Borysko KZ, Wise DS, Wotring LL, Townsend LB (1993) Synthesis of 2,4-disubstituted thiazoles and selenazoles as potential antitumor and antifilarial agents: 1. Methyl 4-(Isothiocyanatomethyl)thiazole-2-carbamates, −selenazole-2-carbamates, and related derivatives. J Med Chem 36:3843–3848
Soriano-Garcia M (2004) Organoselenium compounds as potential therapeutic and chemopreventive agents: a review. Curr Med Chem 11:1657–1669
Mugesh G, du Mont WW, Sies H (2001) Chemistry of biologically important synthetic organoselenium compounds. Chem Rev 101:2125–2180
Nogueira CW, Zeni G, Rocha JBT (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 104:6255–6286
de Silva V, Woznichak MM, Burns KL, Grant KB, May SW (2004) Selenium redox cycling in the protective effects of organoselenides against oxidant-induced DNA damage. J Am Chem Soc 126:2409–2413
Santi C (2014) Organoselenium chemistry: between synthesis and biochemistry. Bentham, Sharjah, UAE
Wirth T (2012) Organoselenium chemistry: synthesis and reactions. Wiley, Weinheim
Jiang X, Lui H (2014) Electrophilic cyclization. In: Knochel, P, Molander, GA (eds) Comprehensive organic synthesis, 2nd edn. Elsevier, Amsterdam, pp 412–494
Kostic MD, Divac VM, Bugarcic ZM (2016) Electrophilic Selenocyclofunctionalization in the synthesis of biologically relevant molecules. Curr Org Chem 20:2606–2619
Santi C, Santoro S (2012) Electrophilic selenium. In: Wirth T (ed) Organoselenium chemistry: synthesis and reactions, 1st edn. Wiley-VCH, Weinheim, pp 1–51
Khokhar SS, Wirth T (2004) Selenocyclizations: control by coordination and by the counterion. Angew Chem Int Ed 43:631–633
Aprile C, Gruttadauria M, Amato ME, Anna FD, Meo PL, Riela S, Noto R (2003) Studies on the stereoselective selenolactonization, hydroxy and methoxy selenenylation of α- and β-hydroxy acids and esters. Synthesis of δ- and γ-lactones. Tetrahedron 59:2241–2251
Petragnani N, Stefani HA, Valduga CJ (2001) Recent advances in selenocyclofunctionalization reactions. Tetrahedron 57:1411–1448
Konstantinovic S, Bugarcic Z, Milosavljevic S, Schroth G, Mihailovic MLJ (1992) Regioselectivity in cyclofunctionalization of olefinic alcohols with benzeneselenenyl halides at different temperatures. Liebigs Ann Chem 23:261–268
Espenson JH (1995) Chemical kinetics and reaction mechanisms, 2nd edn. McGraw Hill, New York
Bugarcic ZM, Mojsilovic BM, Divac VM (2007) Facile pyridine-catalyzed phenylselenoetherification of alkenols. J Mol Catal A Chem 272:288–292
Kostic MD, Divac VM, Puchta R, Bugarcic ZM (2015) Kinetic and mechanistic insight into Lewis base and acid-mediated phenylselenoetherification of 2,6-dimethyl-hept-5-en-2-ol. Struct Chem 26:915–922
Divac VM, Puchta R, Bugarcic ZM (2012) Kinetic and mechanistic studies of base-catalyzed phenylselenoetherification of (Z)- and (E)-hex-4-en-1-ols. J Phys Chem A 116:7783–7790
Bugarčić ZM, Kostić MD, Divac VM (2016) Stereo- and regioselective synthesis of cyclic ethers by means of organoselenium-mediated cyclization of unsaturated alcohols. Curr Org Chem 20:777–797
Divac VM, Bugarčić ZM (2009) Regio- and stereoselectivity in phenylselenoetherification of (Z)- and (E)-Hex-4-en-1-ols. Synthesis 21:3684–3688
Bugarčić ZM, Rvović MD, Divac VM (2009) Based catalyzed phenylselenoetherification of 6-methylhept-5-en-2-ol. ARKIVOC (xiv):135–145
Rvović MD, Divac VM, Puchta R, Bugarčić ZM (2011) Mechanistic investigation of the base-promoted cycloselenoetherification of pent-4-en-1-ol. J Mol Model 17:1251–1257
Clark T, Hennemann M, Murray JS, Politzer PJ (2007) Halogen bonding: the σ-hole. J Mol Model 13:291–296
Murray JS, Lane P, Clark T, Politzer PJ (2007) σ-Hole bonding: molecules containing group VI atoms. J Mol Model 13:1033–1038
Murray JS, Lane P, Politzer PJ (2008) Simultaneous σ-hole and hydrogen bonding by sulfur- and selenium- containing heterocycles. Int J Quantum Chem 108:2770–2781
Politzer PJ, Murray JS, Concha MM (2008) σ-Hole bonding between like atoms; a fallacy of atomic charges. J Mol Model 14:659–665
Politzer P, Murray JS, Clark T, Resnati G (2017) The σ-hole revisited. Phys Chem Chem Phys 19:32166–32178
Acknowledgments
Financial support for this study was obtained from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant: 172011). This research is a part of the scientific network SeS Redox and Catalysis, which deals with multidisciplinary investigation of the chemical, biochemical and pharmaceutical properties of selenium and sulfur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Divac, V.M., Kostić, M.D. & Bugarčić, Z.M. The influence of cobalt(II) and tin(II) chloride on regioselectivity and kinetics of phenylselenocyclization of 6-methyl-hept-5-en-2-ol. J Mol Model 25, 158 (2019). https://doi.org/10.1007/s00894-019-4054-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4054-z