Abstract
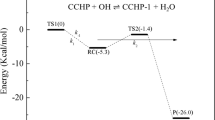
A computational study of cyclohexane autoxidation and catalytic oxidation to a cyclohexyl hydroperoxide intermediate (CyOOH), cyclohexanol, and cyclohexanone has been conducted using a hybrid density functional theory method. The activation of cyclohexane and O2 is the rate-determining step in the formation of CyOOH due to its relatively high energy barrier of 41.2 kcal/mol, and the subsequent reaction behavior of CyOOH controls whether the production of cyclohexanol or cyclohexanone is favored. Using CH3COOH or (CH3COO)2Co as a catalyst reduces the energy barriers required to activate cyclohexane and O2 by 4.1 or 7.9 kcal/mol, respectively. Employing CH3COOH improves the CyOOH intramolecular dehydration process, which favors the formation of cyclohexanone. The energy barrier to the decomposition of CyOOH to CyO·, an important precursor of cyclohexanol, decreases from 35.5 kcal/mol for autoxidation to 25.9 kcal/mol for (CH3COO)2Co catalysis. (CH3COO)2Co promotes the autoxidation process via a radical chain mechanism. The computational results agree with experimental observations quite well, revealing the underlying role of CH3COOH and Co ion in cyclohexane oxidation.

Through DFT analysis of cyclohexane autoxidation and catalytic oxidation, we reveal the mechanism of the effects of CH3COOH and Co2+ on the reaction routes









Similar content being viewed by others
References
Li L, Yang Q, Chen S, Hou X, Liu B, Lu J, Jiang H (2017) Boosting selective oxidation of cyclohexane over a metal-organic framework by hydrophobicity engineering of pore walls. Chem Commun 53:10026–10029
Sakthivel A, Selvam P (2002) Mesoporous (Cr)MCM-41: a mild and efficient heterogeneous catalyst for selective oxidation of cyclohexane. J Catal 211:134–143
Hong Y, Sun D, Fang Y (2018) The highly selective oxidation of cyclohexane to cyclohexanone and cyclohexanol over VAlPO4 berlinite by oxygen under atmospheric pressure. Chem Cent J 12(1):–9
Liu J, Chen T, Yan X, Wang Z, Jian R, Jian P, Yuan E (2018) NiCo2O4 nanoneedle-assembled hierarchical microflowers for highly selective oxidation of styrene. Chem Commun 109:71–75
Buijs W (2003) Challenges in oxidation catalysis. Top Catal 24:73–78
Adamczyk P, Paneth P (2011) Theoretical evaluation of isotopic fractionation factors in oxidation reactions of benzene, phenol and chlorophenols. J Mol Model 17:2285–2296
Xiao Y, Liu J, Xie K, Wang W, Fang Y (2017) Aerobic oxidation of cyclohexane catalyzed by graphene oxide: effects of surface structure and functionalization. Mol Catal 431:1–8
Liu X, Conte M, He Q, Knight DW, Murphy DM, Taylor SH, Whiston K, Kiely CJ, Hutchings GJ (2017) Catalytic partial oxidation of cyclohexane by bimetallic Ag/Pd nanoparticles on magnesium oxide. Chemistry 23:11834–11842
Liu X, Conte M, Sankar M, He Q, Murphy DM, Morgan D, Jenkins RL, Knight D, Whiston K, Kiely CJ, Hutchings GJ (2015) Liquid phase oxidation of cyclohexane using bimetallic Au-Pd/MgO catalysts. Appl Catal A 504:373–380
Retcher B, Costa J, Tang J, Hage R, Gamez P, Reedijk J (2008) Unexpected high oxidation of cyclohexane by Fe salts and dihydrogen peroxide in acetonitrile. J Mol Catal 286:1–5
Ruiter G, Costa J, Lappalainen K, Roubeau O, Gamez P, Reedijk J (2008) The system iron(II)/mpzbpy mediates the H2O2 oxidation of cyclohexane and cyclooctene and the aerobic oxidative cleavage of ascorbic acid to oxalate. Inorg Chem Commun 11:787–790
Maksimchuk N, Kovalenko K, Fedin V, Kholdeeva O (2012) Cyclohexane selective oxidation over metal-organic frameworks of MIL-101 family: superior catalytic activity and selectivity. Chem Commun 48:6812–6814
Jhansi M, Kishore L, Kumar A (2007) Kinetic study of oxidation of cyclohexane using complex catalyst. AICHE J 53:1550–1561
Zhou L, Xu J, Miao H, Wang F, Li X (2005) Catalytic oxidation of cyclohexane to cyclohexanol and cyclohexanone over Co3O4 nanocrystals with molecular oxygen. Appl Catal A 292:223–228
Partenheimer W (1991) Characterization of the reaction of cobalt(II) acetate, dioxygen and acetic acid, and its significance in autoxidation reactions. J Mol Catal A 67:35–46
Vanoppen DL, Devos DE, Genet MJ, Rouxhet PG, Jacobs PA (1995) Cobalt-containing molecular-sieves as catalysts for the low conversion autoxidation of pure cyclohexane. Angew Chem Int Edit 34:560–563
Vereecken L, Nguyen TL, Hermans I, Peeters J (2004) Computational study of the stability of α-hydroperoxyl- or α-alkylperoxyl substituted alkyl radicals. Chem Phys Lett 393:432–436
Lončarević D, Krstić J, Dostanić J, Manojlović D, Čupić Ž, Jovanović DM (2010) Cyclohexane oxidation and cyclohexyl hydroperoxide decomposition by poly(4-vinylpyridine-co-divinylbenzene) supported cobalt and chromium complexes. Chem Eng J 157:181–188
Zhang Y, Li Z, Sun W, Xia C (2009) Co-doped attapulgite catalyzed solvent-free oxidation of cyclohexane using molecular oxygen. Catal Lett 129:222–227
Yuan HX, Xia QH, Zhan HJ, Lu XH, Su KX (2006) Catalytic oxidation of cyclohexane to cyclohexanone and cyclohexanol by oxygen in a solvent-free system over metal-containing ZSM-5 catalysts. Appl Catal A 304:178–184
Li J, Shi Y, Xu L, Lu G (2010) Selective oxidation of cyclohexane over transition-metal-incorporated HMS in a solvent-free system. Ind Eng Chem Res 49:5392–5399
Sreevardhan Reddy S, David Raju B, Padmasri AH, Sai Prakash PK, Rama Rao KS (2009) Novel and efficient cobalt encapsulated SBA-15 catalysts for the selective oxidation of cyclohexane. Catal Today 141:61–65
Li L, Li H, Jin C, Wang X, Ji W, Pan Y, van der Knaap T, van der Stoel R, Au CT (2010) Surface cobalt silicate and CoOx cluster anchored to SBA-15: highly efficient for cyclohexane partial oxidation. Catal Lett 136:20–27
Tan X, Wang X, Liu Q, Zhou J, Zhang P, Zheng S, Miao S (2017) Bio-gel template synthesis of CoFe2O4 nano-catalysts and application in aerobic oxidation of cyclohexane. Int J Hydrogen Energ 42:19001–19009
Tong J, Bo L, Li Z, Lei Z, Xia C (2009) Magnetic CoFe2O4 nanocrystal: a novel and efficient heterogeneous catalyst for aerobic oxidation of cyclohexane. J Mol Catal A 307:58–63
Unnarkat AP, Sridhar T, Wang H, Mahajani SM, Suresh AK (2018) Study of cobalt molybdenum oxide supported on mesoporous silica for liquid phase cyclohexane oxidation. Catal Today 310:116–129
Wu M, Zhan W, Guo Y, Guo Y, Wang Y, Wang L, Lu G (2016) An effective Mn-Co mixed oxide catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen. Appl Catal A 523:97–106
Hermans I, Peeters J, Jacobs PA (2006) Enhanced activity and selectivity in cyclohexane autoxidation by inert H-bond acceptor catalysts. Chemphyschem 7:1142–1148
Murahashi S, Oda Y, Naota T (1992) Iron- and ruthenium-catalyzed oxidations of alkanes with molecular oxygen in the presence of aldehydes and acids. J Am Chem Soc 114:7913–7914
Hereijgers BPC, Weckhuysen BM (2010) Aerobic oxidation of cyclohexane by gold-based catalysts: new mechanistic insight by thorough product analysis. J Catal 270:16–25
Hermans I, Jacobs PA, Peeters J (2006) Understanding the autoxidation of hydrocarbons at the molecular level and consequences for catalysis. J Mol Catal A 251:221–228
Hermans I, Jacobs P, Peeters J (2007) The formation of byproducts in the autoxidation of cyclohexane. Chemistry 13:754–761
Terán J, Zambrano C, Mora J, Rincón L, Torres FJ (2018) Theoretical investigation of the mechanism for the reductive dehalogenation of methyl halides mediated by the CoI-based compounds cobalamin and cobaloxime. J Mol Model 24:316
Yuan E, Wang L, Zhang X, Feng R, Wu C, Li G (2016) Density functional theory analysis of anthraquinone derivative hydrogenation over palladium catalyst. Chemphyschem 17:3974–3984
Roy LE, Hay PJ, Martin RL (2008) Revised basis sets for the LANL effective core potentials. J Chem Theory Comput 4:1029–1031
Wong MW, Frisch MJ, Wiberg KB (1991) Solvent effects. 1. The mediation of electrostatic effects by solvents. J Am Chem Soc 113:4776–4782
Frisch MJ, Trucks GW, Schlegel HB, et al. (2009) Gaussian 09, revision A.01. Gaussian, Inc., Wallingford
Hermans I, Nguyen TL, Jacobs PA, Peeters J (2005) Autoxidation of cyclohexane: conventional views challenged by theory and experiment. Chemphyschem 6:637–645
Salem IA, El-Maazawi M, Zaki AB (2000) Kinetics and mechanisms of decomposition reaction of hydrogen peroxide in presence of metal complexes. In J Chem Kinet 32:643–666
Conte M, Liu X, Murphy DM, Whiston K, Hutchings GJ (2012) Cyclohexane oxidation using Au/MgO: an investigation of the reaction mechanism. Phys Chem Chem Phys 14:16279–16285
Buijs W, Raja R, Thomas J, Wolters H (2003) On the similarity in catalytic activity of homogeneous and heterogeneous Cr(VI) catalysts in the decomposition of cyclohexyl hydroperoxide. Catal Lett 91:253–259
Hamann J, Hermsen M, Schmidt A, Krieg S, Schießl J, Riedel D, Teles J, Schäefer A, Comba P, Hashmi A, Schaub T (2018) Selective decomposition of cyclohexyl hydroperoxide using homogeneous and heterogeneous Cr(VI) catalysts: optimizing the reaction by evaluating the reaction mechanism. ChemCatChem 10:2755–2767. https://doi.org/10.1002/cctc.201701909
Kharkova TV, Arest-Yakubovich IL, Lipes VV (1989) Kinetic model of the liquid-phase oxidation of cyclohexane. I. Homogeneous proceeding of the process. Kinet Catal 30:954–958
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20180935), the Natural Science Foundation for High Education of Jiangsu Province (17KJB530011), and the Science and Technology Innovation Foundation of Yangzhou University (2017CXJ015).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1.
Structures and energies of (CH3COO)2Co and CyOOH conformers. Reaction energy diagram for the dissociation of the O–H bond in CyOOH, the formation of cyclohexanone via the intramolecular dehydration of CyOOH, the autoxidation of cyclohexane with the direct involvement of one cyclohexane molecule, the reaction between two CyOO· radicals, the formation of CyOH via the oxidation of CyH by CyOOH, catalytic dehydration of CyOOH over Q=O, and the catalytic decomposition of CyOOH over CH3COOH. The Cartesian coordinates of all minima and transition states in the reaction routes are also shown. (DOCX 956 kb)
Rights and permissions
About this article
Cite this article
Yuan, E., Liu, H., Tao, Y. et al. Density functional theory study of selective aerobic oxidation of cyclohexane: the roles of acetic acid and cobalt ion. J Mol Model 25, 71 (2019). https://doi.org/10.1007/s00894-019-3949-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-3949-z