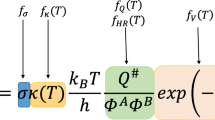Abstract
Hydrogen abstraction from carbonyl-hydroperoxide is a new reaction class in the low-temperature oxidation of hydrocarbons. In this work, a comprehensive study to the kinetics for the hydrogen abstraction from cyclohexane-carbonyl-hydroperoxide (CCHP) is investigated using the CBS-QB3 composite method. Five small active radicals (H, CH3, O (3P), OH and HO2) are selected as the extracting agents, and the corresponding barrier heights are computed. Guided by the reaction barriers, the preferable path on hydrogen abstraction from CCHP is identified. The two-transition-state model is employed to obtain the overall rate constant when HO2 and OH act as the extracting agents due to the formation of reactant and product complexes. High-pressure-limit rate constants for 25 elementary reactions are reported in the modified Arrhenius form. Branching ratios for the site-specific hydrogen abstraction reactions ranging from 300 to 2500 K are illustrated to show the temperature dependence of preferable path. Compared with the theoretical rate constants obtained in this work, the values estimated by using analogy rules have obvious deviations at low temperature. The obtained hydrogen abstraction reactions are added to the JetSurF2.0 mechanism, thereby improving its kinetic modeling results for cyclohexane oxidation. Present work provides accurate kinetic parameters for this new type of reaction class which can be helpful to improve the predictive capability for hydrocarbon mechanism.









Similar content being viewed by others
References
Herbinet O, Battin-Leclerc F, Bax S, Gall HL, Glaude PA, Fournet R, Zhou Z, Deng L, Guo H, Xie M, Qi F (2011) Phys Chem Chem Phys 13(1):296
Herbinet O, Husson B, Serinyel Z, Cord M, Warth V, Fournet R, Glaude PA, Sirjean B, Battin-Leclerc F, Wang Z, Xie M, Cheng Z, Qi F (2012) Combust Flame 159(12):3455
Ranzi E, Cavallotti C, Cuoci A, Frassoldati A, Pelucchi M, Faravelli T (2015) Combust Flame 162(5):1679
Pelucchi M, Bissoli M, Cavallotti C, Cuoci A, Faravelli T, Frassoldati A, Ranzi E, Stagni A (2014) Energy Fuels 28(11):7178
Zhou CW, Li Y, O’Connor E, Somers KP, Thion S, Keesee C, Mathieu O, Petersen EL, DeVerter TA, Oehlschlaeger MA, Kukkadapu G, Sung C-J, Alrefae M, Khaled F, Farooq A, Dirrenberger P, Glaude PA, Battin-Leclerc F, Santner J, Ju Y, Held T, Haas FM, Dryer FL, Curran HJ (2016) Combust Flame 167:353
Sirjean B, Glaude PA, Ruiz-Lòpez MF, Fournet R (2009) J Phys Chem A 113(25):6924
Burke S, Burke U, Donagh R, Mathieu O, Osorio I, Keesee C, Morones A, Petersen E, Wang W, DeVerter T, Oehlschlaeger M, Rhodes B, Hanson RK, Davidson D, Weber B, Sung C-J, Santner J, Ju Y, Haas F, Curran HJ (2015) Combust Flame 162(2):296
Keromnes A, Metcalfe W, Heufer A, Donohoe N, Das A, Sung C-J, Herzler J, Naumann C, Griebel P, Mathieu O, Krejci M, Petersen E, Pitz W, Curran HJ (2013) Combust Flame 160(6):995
Franklin Goldsmith C, Green W, Klippenstein S (2012) J Phys Chem A 116(13):3325
Xing L, Zhang L, Zhang F, Jiang J (2017) Combust Flame 182:216
Antonov I, Zádor J, Rotavera B, Papajak E, Osborn D, Taatjes C, Sheps L (2016) J Phys Chem A 120(33):6582
Ranzi E, Dente M, Faravelli T, Pennati G (1993) Combust Sci Technol 95:1
Rossi M (2017) Atmos Chem Phys 13(15):7359
Silke EJ, Pitz WJ, Westbrook CK, Ribaucour M (2007) J Phys Chem A 111(19):3761
Bakali AE, Braun-Unkhoff M, Dagaut P, Frank P, Cathonnet M (2000) Proc Combust Inst 28(2):1631
Serinyel Z, Herbinet O, Frottier O, Dirrenberger P, Warth V, Glaude PA, Battin-Leclerc F (2013) Combust Flame 160(11):2319
Knepp AM, Meloni G, Jusinski LE, Taatjes CA, Cavallotti C, Klippenstein SJ (2007) Phys Chem Chem Phys 9(31):4315
B Sirjean, E Dames, DA Sheen, FN Egolfopoulos, H.Wang, DF Davidson, RK Hanson, H Pitsch, CT Bowman, CK Law, W Tsang, NP Cernan-sky, DL Miller, A Violi, RP Lindstedt (2010) JetSurF version2.0, Septem-ber19, http://melchior.usc.edu/JetSurF/JetSurF2.0
Montgomery J, Frisch M, Ochterski J, Petersson GA (1999) J Chem Phys 110(6):2822
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA,Cheeseman JR, Scalmani G, Barone V, Mennucci B, PeterssonGA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF,Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, KitaoO, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, OgliaroF, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, GompertsR, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkaso, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian09 Revision B.01. Gaussian Inc, Wallingford
Sirjean B, Glaude PA, Ruiz-Lopez M (2006) J Phys Chem A 110(46):12693
Sirjean B, Fournet R (2012) J Phys Chem A 116(25):6675
Altarawneh M, Dlugogorski B, Kennedy E, Mackie J (2013) Combust Flame 160(1):9
Ning H, Gong C, Tan N, Li Z, Li X (2015) Combust Flame 162(11):4167
Gonzalez C, Bernhard Schlegel H (1989) J Chem Phys 90(90):2154
Curtiss LA, Raghavachari K, Redfern P, Pople JA (1997) J Chem Phys 106(3):1063–1079
Mokrushin V, Tsang W (2009) ChemRate v158, National Institue of Standards and Technology: Gaithersburg M 2009, Chemrate 2009
Johnston HS, Heicklen J (1962) J Phys Chem 66(3):532
Tardy DC, Rabinovitch BS (1977) Chem Rev 77(3):369
Wang H, Frenklach M (1994) Combust Flame 96(1–2):163
Pechukas P (1981) Annu Rev Phys Chem 32(32):59
Tan T, Yang X, Ju Y, Carter A (2016) Phys Chem Chem Phys 18(6):4594
Li SH, Guo JJ, Li R, Wang F, Li XY (2016) J Phys Chem A 120(20):3424
Tan T, Pavone M, Krisiloff D, Carter A (2012) J Phys Chem A 116(33):8431
Jj W, Khaled F, Hong-Bo N, Ma L, Farooq A, Ren W (2017) J Phys Chem A 121(33):6304
Shannon RJ, Taylor S, Goddard A, Blitz M, Heard D (2010) Phys Chem Chem Phys 12(41):13511
Greenwald E, North S, Georgievskii Y, Klippenstein JS (2007) J Phys Chem A 111(25):5582
Klippenstein J (1992) J Chem Phys 96(7):5558
Klippenstein J (1994) J Phys Chem 98(44):11459
Curran HJ, Gaffuri P, Pitz W, Westbrook CK (2002) Combust Flame 129(3):253
CHEMKIN-PRO 15092 Reaction Design: San Diego 2009
Acknowledgements
This work is supported by the National Science Foundation of China (Nos. 91741201, 91641120).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tu, Y., Wang, JB. & Li, XY. Theoretical study of hydrogen abstraction by small radicals from cyclohexane-carbonyl-hydroperoxide. Theor Chem Acc 138, 39 (2019). https://doi.org/10.1007/s00214-019-2426-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2426-1