Abstract
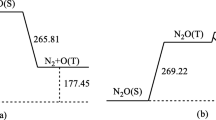
The reactions between arsenic and nitrogen oxides (N2O, NO2, and NO) were investigated using density functional theory. The geometries of the reactants, intermediates, transition states, and products in each reaction were optimized. Frequency analysis was applied to verify those geometries, and the authenticity of each transition state was checked using intrinsic reaction coordinate analysis (IRC). The single point energy of each stationary point was calculated at the B2PLYP level, and kinetic analysis was conducted to explore each reaction mechanism in more detail. Results showed that the energy barriers to the reactions of As with N2O, NO2, and NO were 78.45, 2.58, and 155.85 kJ mol−1, respectively. For each reaction, the rate increased as the temperature was increased from 298 to 1800 K. However, temperature had only a tiny impact on the reaction of As with NO2 due to the low energy barrier involved, and the reaction rate was consistently high (>1012 cm3 mol−1 s−1), which indicates that this reaction occurs readily. On the other hand, the rate of the reaction between As and N2O or NO increased rapidly between 298 and 900 K, and then increased more gradually upon further increasing the temperature.





Similar content being viewed by others
References
Liu G, Zheng L, Duzgoren-Aydin NS, Gao L, Liu J, Peng Z (2007) Health effects of arsenic, fluorine, and selenium from indoor burning of Chinese coal. Rev Environ Contam Toxicol 189:89–106
Zhao Y, Zhang J, Huang W, Wang Z, Li Y, Song D, Zhao F, Zheng C (2008) Arsenic emission during combustion of high arsenic coals from southwestern Guizhou, China. Energy Convers Manag 49(4):615–624
Jiao F, Ninomiya Y, Zhang L, Yamada N, Sato A (2013) Effect of coal blending on the leaching characteristics of arsenic in fly ash from fluidized bed coal combustion. Fuel Process Technol 106:769–775
Shah P, Strezov V, Prince K, Nelson PF (2008) Speciation of As, Cr, Se and Hg under coal fired power station conditions. Fuel 87(10-11):1859–1869
Wang C, Liu H, Zhang Y, Zou C, Anthony EJ (2018) Review of arsenic behavior during coal combustion: volatilization, transformation, emission and removal technologies. Prog Energy Combust Sci 68:1–28
Tang Q, Liu G, Yan Z, Sun R (2012) Distribution and fate of environmentally sensitive elements (arsenic, mercury, stibium and selenium) in coal-fired power plants at Huainan, Anhui, China. Fuel 95:334–339
Liu H, Pan W, Wang C, Zhang Y (2016) Volatilization of arsenic during coal combustion based on isothermal thermogravimetric analysis at 600–1500 °C. Energy Fuel 30(8):6790–6798
Liu H, Wang C, Sun X, Zhang Y, Zou C (2016) Volatilization of arsenic in coal during isothermal oxy-fuel combustion. Energy Fuel 30(4):3479–3487
Contreras ML, Arostegui JM, Armesto L (2009) Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations. Fuel 88(3):539–546
Frandsen F, Johansen D, Rasmussen P (1994) Trace elements from combustion and gasification of coal—an equilibrium approach. Prog Energy Combust Sci 20(2):115–138
Urban DR, Wilcox J (2006) A theoretical study of properties and reactions involving arsenic and selenium compounds present in coal combustion flue gases. J Phys Chem A 110(17):5847–5852
Monahan-Pendergast M, Przybylek M, Lindblad M, Wilcox J (2008) Theoretical predictions of arsenic and selenium species under atmospheric conditions. Atmos Environ 42(10):2349–2357
Xu J, Sun R, Ismail TM, Sun S, Wang Z (2017) Effect of char particle size on NO release during coal char combustion. Energy Fuel 31(12):13406–13415
Wang Z, Xu J, Sun R, Zhao Y, Li Y, Ismail TM (2017) Investigation of the NO reduction characteristics of coal char at different conversion degrees under an NO atmosphere. Energy Fuel 31:8722–8732
Zhang H, Liu J, Shen J, Jiang X (2015) Thermodynamic and kinetic evaluation of the reaction between NO (nitric oxide) and char(N) (char bound nitrogen) in coal combustion. Energy 82:312–321
Liu J, Qu W, Yuan J, Wang S, Qiu J, Zheng C (2010) Theoretical studies of properties and reactions involving mercury species present in combustion flue gases. Energy Fuel 24(1):117–122
Peng Y, Li J, Si W, Luo J, Dai Q, Luo X, Liu X, Hao J (2014) Insight into deactivation of commercial SCR catalyst by arsenic: an experiment and DFT study. Environ Sci Technol 48(23):13895–13900. https://doi.org/10.1021/es503486w
Liu J, Qu W, Zheng C (2013) Theoretical studies of mercury–bromine species adsorption mechanism on carbonaceous surface. Proc Combust Inst 34(2):2811–2819
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Du X, Tang J, Gao X, Chen Y, Ran J, Zhang L (2016) Molecular transformations of arsenic species in the flue gas of typical power plants: a density functional theory study. Energy Fuel 30(5):4209–4214
Awuaha JB, Dzade NY, Tiac R, Adeic E, Kwakye-Awuahad B, Catlowe CRA, Leeuwbde NH (2016) A density functional theory study of arsenic immobilization by the Al(III)-modified zeolite clinoptilolite. Phys Chem Chem Phys 18(16):11297–11305
Gao Z, Yang W (2016) Effects of CO/CO2/NO on elemental lead adsorption on carbonaceous surfaces. J Mol Model 22:166
Ye S, Neese F (2010) Accurate modeling of spin-state energetics in spin-crossover systems with modern density functional theory. Inorg Chem 49:772–774
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, revision D.01. Gaussian, Inc., Wallingford
Gao Z, Lv S, Yang W, Yang P, Ji S, Meng X (2015) Quantum chemistry investigation on the reaction mechanism of the elemental mercury, chlorine, bromine and ozone system. J Mol Model 21:160
Schröder B, Sebald P, Stein C, Weser O, Botschwina P (2015) Challenging high-level ab initio rovibrational spectroscopy: the nitrous oxide molecule. Z Phys Chem 229(10-12):1663–1690
Borisenko KB, Kolonits R, Rozsondai B, Hargittai I (1997) Electron diffraction study of the nitrogen dioxide molecular structure at 294, 480, and 691 K. J Mol Struct 413-414:121–131
Marsden CJ, Smith BJ (1989) Ab initio force constants: a cautionary tale concerning nitrogen oxides. J Mol Struct THEOCHEM 187:337–357
Evenson KM, Wells JS, Radford HE (1970) Infrared resonance of OH with the H2O laser: a galactic maser pump? Phys Rev Lett 25(4):199–202
Mizushima M (1972) Molecular parameters of OH free radical. Phys Rev A 5(1):143–157
Acknowledgements
Financial support was provided by the National Key R&D Program of China (no. 2016YFB0600701).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, C., Wang, C. Theoretical study of the reactions between arsenic and nitrogen oxides during coal combustion. J Mol Model 25, 30 (2019). https://doi.org/10.1007/s00894-018-3916-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3916-0