Abstract
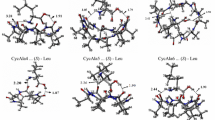
Gamma-valerolactone (GVL) is a cyclic ester that can be considered a green alternative in chemical processes due to its environmentally friendly physical and chemical properties and low production cost from biomass. Although GVL is a chiral solvent, it is usually used as a racemic mixture, instead of its homochiral forms, which might improve its performance in enantioselective synthesis and chiral separation chromatographic techniques. This report presents the development and validation of an atomistic force field optimized to reproduce GVL liquid-phase properties via Monte Carlo (MC) and molecular dynamics (MD) simulation methods. The optimized force field improved the description of the interactions between pairs of molecules, which is a key aspect for a proper assessment of subtle interactions between the enantiomeric forms of GVL. Inspection of radial distribution functions (RDF) for correlations between RR, SS, and RS interactions found within GVL racemic mixture shows very subtle differences at the first solvation shell. Average interaction energies \(E_{int}^{RR}\), \(E_{int}^{SS}\) and \(E_{int}^{RS}\) for RR, SS, and RS dimer ensembles, respectively, were calculated with force field and also HF-3c and PBEh-3c quantum chemistry methods. For each methodology, resulting values obtained for \(E_{int}^{RR}\) and \(E_{int}^{SS}\) were almost the same and more negative than \(E_{int}^{RS}\). Also, the average energy fluctuation obtained for RR and SS dimers were higher than the one obtained for RS.







Similar content being viewed by others
References
Capello C, Fischer U, Hungerbühler K (2007) Green Chem 9:927
Serrano-Ruiz JC, Pineda A, Balu AM, Luque R, Campelo JM, Romero AA, Ramos-fernández JM (2012) Catal Today 195:162
Horváth IT, Mehdi H, Fábos V, Boda L, Mika LT (2008) Green Chem 10:238
Marinetti LJ, Leavell BJ, Jones CM, Hepler BR, Isenschmid DS, Commissaris RL (2012) Pharmacol Biochem Behav 101:602
Alonso DM, Wettstein SG, Dumesic JA (2013) Green Chem 15:584
Qi L, Horváth IT (2012) ACS Catal 2:2247
Qi L, Mui YF, Lo SW, Lui MY, Akien GR, Horváth IT (2014) ACS Catalysis 4:1470
Fegyverneki D, Orha L, Láng G, Horváth IT (2010) Tetrahedron 66:1078
Tomioka K, Ishiguro T, Koga K (1980) Tetrahedron Lett 21:2973
Gorissen HJ, Hoeck JPV, Mockel AM, Journée GH, Delatour C, Libert VR (1992) Chirality 4:286
Baldwin JE, Adlington RM, Ramcharitar SH (1992) Synlett 1992:875
Stangeland EL, Sammakia T (2004) J Org Chem 69:2381
Mori K (1975) Tetrahedron 31:3011
Koerwitz FL, Hammond GB, Wiemer DF (1989) J Org Chem 54:738
Tukacs JM, Fridrich B, Dibó G, Székely E, Mika LT (2015) Green Chem 17:5189
Aparicio S, Alcalde R (2009) Phys Chem Chem Phys 11:6455
Havasi D, Mizsey P, Mika LT (2016) J Chem Eng Data 61:1502
Klajmon M, Řehák K, Matoušová M, Morávek P (2016) J Chem Eng Data 61:391
Zaitseva A, Pokki JP, Le HQ, Alopaeus V, Sixta H (2016) J Chem Eng Data 61:881
Schaftenaar G, Noordik JH (2000) J Comput Aided Mol Des 14:123
Neese F (2012) Wiley Interdiscip Rev: Comput Mol Sci 2:73
Becke AD (1988) Phys Rev A 38:3098
Perdew J (1986) Phys Rev B 33:8822
Schäfer A, Horn H, Ahlrichs R (1992) J Chem Phys 97:2571
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297
Wachters AJH (1970) J Chem Phys 52:1033
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100(8):5829–5835. https://doi.org/10.1063/1.467146
Kendall RA, Früchtl HA (1997) Theor Chem Acc 97:158
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) J Am Chem Soc 118:11225
Berendsen H, van der Spoel D, van Drunen R (1995) Comput Phys Commun 91:43
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) J Comput Chem 26:1701
Pronk S, Pall S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E (2013) Bioinformatics 29:845
Breneman CM, Wiberg KB (1990) J Comput Chem 11:361
Lide DR (2009) CRC handbook of chemistry and physics, 90th edn, chap 3. CRC Press, Cleveland
Emel’yanenko VN, Kozlova SA, Verevkin SP, Roganov GN (2008) J Chem Thermodyn 40:911
Freitas LCG (2009) J Braz Chem Soc 20:1541
Freitas LCG, Cordeiro JMM (1995) J Mol Struct: THEOCHEM 335:189
Freitas LCG, Cordeiro JMM, Garbujo FLL (1999) J Mol Liq 79:1
Belletato P, Freitas LCG, Arêas EPG, Santos PS (1999) Phys Chem Chem Phys 1:4769
Caleman C, van Maaren PJ, Hong M, Hub JS, Costa LT, van der Spoel D (2012) J Chem Theory Comput 8:61
Wang J, Hou T (2011) J Chem Theory Comput 7:2151
Price MLP, Ostrovsky D, Jorgensen WL (2001) J Comput Chem 22:1340
Stewart JJP (2012) J Mol Model 19(1):1
MOPAC2012, Stewart JPP (2012) Stewart Computational Chemistry, Colorado Springs, CO, USA HTTP://OpenMOPAC.net
Davidson N (1962) Statistical mechanics. McGraw-Hill Book Company, New York
Craig DP, Mellor DP (1976) Top Curr Chem 0:1
Portmann S, Inauen A, Lüthi HP, Leutwyler S (2000) J Chem Phys 113:9577
Su Z, Borho N, Xu Y (2006) J Am Chem Soc 128:17126
Garten S, Biedermann PU, Topiol S, Agranat I (2009) Electrostatic chiral distinction: Tetrahedral model dimers. Chirality 22(7):662–674
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32:1456
Kruse H, Grimme S (2012) J Chem Phys 136:154101
Sure R, Grimme S (2013) J Comput Chem 34:1672
Grimme S, Brandenburg JG, Bannwarth C, Hansen A (2015) J Chem Phys 143(5):054107
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry, 4th ed. Weinheim:Wiley-VCH, New York
North M, Villuendas P (2010) Org Lett 12:2378
Tulashie SK, von Langermann J, Lorenz H, Seidel-Morgenstern A (2011) Cryst Growth Des 11:240
Acknowledgements
The authors are grateful to the Brazilian funding agencies CAPES, CNPq-INCT (573742/2008-1) and FAPESP (2012/15147-4 and 2013/072962) for financial support. FMC is grateful to CNPq for the award of a scholarship and AFM is grateful to MEC/PET for a fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supporting information available: A file is provided with a detailed description of the GVL structure and force-field parameters used in this paper.
This paper belongs to Topical Collection XIX - Brazilian Symposium of Theoretical Chemistry (SBQT2017)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Colombari, F.M., de Moura, A.F. & Freitas, L.C.G. Chiral recognition of liquid phase dimers from gamma-valerolactone racemic mixture. J Mol Model 24, 215 (2018). https://doi.org/10.1007/s00894-018-3744-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3744-2