Abstract
Our investigation is devoted to the theoretical study of the low-lying electronic structure of the LaCl molecule by using ab initio quantum methods. We are concerned with several methods such as the complete active space-self consistent field (CAS-SCF) and the multi reference of configuration interaction (MRCI + Q) methods. These methods are applied for the purpose of drawing the potential energy curves (PECs) and calculating the molecular spectroscopic constants for a given number of electronic states of singlet and triplet multiplicity. We count 26 2S+ 1 Λ(±) electronic states located below 24,000 cm− 1 neglecting the spin-orbit effects and 47 Ω(±) components taken into consideration these effects. Our calculations are performed via the quantum ab initio package MOLPRO (Werner and Knowles 2000).

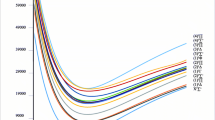
A new set of low-lying electronic states on the theoretical energetic level diagram for the LaCl molecule among the first four lanthanum monhalides.








Similar content being viewed by others
References
Werner H-J, Knowles P-J (2000) MOLPRO ab-initio program package. Universities of Stuttgart and Birmingham, Birmingham
Xiaoyan C, Wenjian L, Dolg M (2002) Molecular structure of diatomic lanthanide compounds. Science In China (series B) 45(1):91–96
Xiaoyan C, Dolg M (2005) Pseudopotential studies on the electronic structure of lanthanum monohalides LaF, LaCl LaBr, and LaI. J Theor Comput Chem 04 spec01:583–592
Field RW (1982) Diatomic molecule electronic structure beyond simple molecular constants. Ber Bunsenges Phys Chem 86:771–779
Linton CS, Rice MS, Dulick M et al (1983) Laser spectroscopy of YbO: observation and analysis of some 0+-1 Σ+ transitions. J Mol Spectrosc 101(1):332–343
Dulick M, Murad E, Barrow RF (1986) Thermochemical properties of the rare earth monoxide. J Chem Phys 85(1):385– 390
McDonald SA, Rice RF, Field RW (1990) Laser spectroscopy of low-lying excited states in YbO: linkage of the Y b 2+ f 13 s and f 14 configurations. J Chem Phys 93(11):7676–7686
Dolg M, Stoll H (1996) Electronic structure calculations for molecules containing lanthanide atoms. In: Gschneidner KA Jr, Eyring L (eds) Handbook of the physics and chemistry of rare earths, vol 22. Elsevier, Amsterdam, pp 607–729
Chen L-H, Shang R-C (2003) Analytical potential energy function for the ground state X1 Σ+ of LaCl. J Mol Struct, THEOCHEM 629(1–3):37–42
Armentrout PB, Beauchamp JL (1989) The chemistry of atomic transition-metal ions: insight into fundamental aspects of organometallic chemistry. Acc Chem Res 22(9):315–321
Carlson KD, Claydon CR (1967) Electronic structure of molecules of high temperature interest. Adv High Temp Chem 1:43–94
Rubinoff DS, Evans CJ, Gerry MCL (2003) The pure rotational spectra of the lanthanum monohalides, LaF, LaCl, LaBr, LaI. J Mol Spectrosc 218:169–179
Schall H, Linton C, Field RW (1983) Laser spectroscopy of LaF: determination of the separation of the singlet and triplet state manifolds. J Mol Spectrosc 100:437–448
Langhoff SR, Bauschlicher CW Jr, Partridge H (1988) Erratum: Theoretical study of the scandium and yttrium halides. J Chem Phys 89(1):396–407
Xin J, Klynning L (1994) Fourier transform spectroscopy of LaCl: rotational analyses of the infrared band system. Physica Script 49:209–213
Basics R, Bernard AC, Zgainsky A (1975) Electronic spectrum of the lanthanum hydride and lanthanum deuteride molecules. R Acad Sci B, Comptes Rendus des Seances de l’Academie des Sciences, Serie B: Sciences Physiques 280(4):77. 8CODEN: CHDBAN; ISSN: 0366-6077
Barrow RF, Bastin MW, Moore DLG, Pott CJ (1967) Electronic states of gaseous fluorides of scandium, yttrium and lanthanum. Nature 215(5105):1072–1073
Bernard A, Effantin C, d’Incan J, Verges J (2000) The (1)1 π, (2)1 σ + x 1 σ + transitions of LaF. J Mol Spectrosc 202(1):163–165
Kaledin LA, Kaledin AL, Heaven MC (1997) Laser absorption spectroscopy of LaF analysis of the b 1 π − x 1 σ + transition. J Mol Spectrosc 182:50–56
Kaledin LA, Mccord JE, Heaven MC (1994) Laser spectroscopy of LaF: ligand field-theory assignment of the triplet-state manifold and analysis of hyperfine structure. J Opt Soc Am B 11:219– 224
Kuchle W, Dolg M, Stoll H (1997) Ab initio study of the lanthanide and actinide contraction. J Phys Chem 101:7128– 7133
Hong GY, Dolg M, Li LM (2001) A comparison of scalar-relativistic ZORA and DKH density functional schemes: monohydrides, monoxides and monofluorides of La, Lu, Ac and Lr. Chem Phys Lett 334:396–402
Laerdahl JK, KF Jr, Visscher L, Saue T (1998) A fully relativistic Dirac–Hartree–Fock and second-order Mller-Plesset study of the lanthanide and actinide contraction. J Chem Phys 109(24):10806–10817
Chervonnyi AD, Chervonnaya NA (2004) Thermodynamic properties of lanthanum chlorides. Inorg Mater 40:1097–1104
Chervonnyi AD, Chervonnaya NA (2007) Thermodynamic properties of some lanthanum and lanthanides, halides: I. Thermodynamic functions of LaF(gas) and LaCl(gas). Russ J Inorg Chem 52(6):884–894
Chervonnyi AD, Chervonnaya NA (2007) Thermodynamic properties of some lanthanum and lanthanide halides: II. Thermodynamic functions of gaseous molecules LnX(Ln=Ce-Lu, X=F, Cl). Russ J Inorg Chem 52(8):1230–1242
Chervonnyi AD, Chervonnaya NA (2007) Thermodynamic properties of lanthanum and lanthanide halides: IV. Enthalpies of atomization of LnCl, LnCl+, LnF, LnF+, and L n F 2. Russ J Inorg Chem 52(12):1937–1952
Kaledin LA, Heaven MC, Field RW (1999) Thermochemical properties (D\(_{0}^{\circ }\) and IP) of the lanthanide monohalides. J Mol Spectrosc 193(2):285–292
Sergeev DN, Motalov VB, Butman MF, Kiselev AE, Kudin LS, Kramer KW (2014) Atomization energies of LnX molecules (Ln = Sm, Eu, and Yb; X = Cl, Br, and I). J Chem Eng data 59:4010–4014
Hamade Y, Taher F, Choueib M, Monteil Y (2009) Theoretical electronic investigation of the low-lying electronic states of LuF molecule. Can. J. Phys 87:1163–1169
Hamade Y, Bazzi H, Sidawi J, Taher F, Monteil Y (2012) Ab initio study of the lowest-lying electronic states of LuCl molecules. J Phys Chem A 116:12123–12128
Fahes H, Korek M, Allouche AR, Aubert-Frecon M (2004) Theoretical electronic structure of the lowest-lying states of the LaCl molecules. J Chem Phys 229:97–103
Davidson ER, Silver DW (1977) Size consistency in the dilute helium gas electronic structure. Chem Phys Lett 52(3):403–406
Martin WC, Zalubas R, Hagan L (1978) Atomic Energy levels. The Rare-Earth Elements, NSRDS-NBRDS60. Nat Stand Ref Data Ser, Nat Bur Stand 60
Zhu ZH (2007) The atomic and molecular reaction statics. Sci China Ser G Phys Mech Astron 50(5):581–590
Murrell JN, Sorbie KS (1974) New analytic from for the potential energy curves of stable diatomic state. J Chem Soc, Faraday Trans II 70:1552–1557
Martin WC, Zalubas R, Hagan L (1978) Nat Stand Ref Data Ser, NSRDS-NBS60, 422 pp Nat Bur Stand
Radziemski LJ, Kaufman V (1969) Wavelengths, energy levels, and analysis of neutral atomic chlorine (Cl I). J Opt Soc Am 59(4):424–443
Stevens WJ, Krauss M, Basch H, Jasien PG (1992) Can J Chem 70:612–630
Cao X, Dolg M (2001) J Chem Phys 115:7348–7355
Bergner A, Dolg M, Kuechle W, Stoll H, Preuss H (1993) Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol Phys 80:1431–1441
Widmark PO, Malmqvist PA, Roos B (1990) Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions. Theor Chem Acta 77(5):291–306
Widmark PO, Persson BJ, Roos B (1991) Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions. Theor Chem Acta 79(6):419–432
Martin JDD, Hepburn JW (1988) Determination of bond dissociation energies by threshold ion-pair production spectroscopy. An improved D-O(HCl). J Chem Phys 109(19):8139–8142
Fahes H, Allouche AR (2002) Theoretical electronic structure of the lowest-lying states of the LaF molecule. J Chem Phys 117:3715–3720
Taher-Mansour F, Allouche AR, Aubert-Frecon M (2003) Theoretical electronic structure of the lowest-lying states of the LaI molecules. J Mol Spectro 221:1–6
Acknowledgements
We present our appreciation and deep thanks to Mr. Florent REAL, Professor at the University of Sciences and Technologies Lille 1-France and member on PHLAM laboratory for his support and sincere discussion of this work. We also thank Pr. Fadia TAHER, Head of the Common trunc at the Faculty of Engineering III on the Lebanese University and the director of the Molecular Quantum Mechanics and Modeling Laboratory. We also thank Mr. Jihad SIDAWI, Professor at the Faculty of Engineering III at the Lebanese University and member on the Molecular Quantum Mechanics and Modeling Laboratory for his cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamade, Y., El Sobbahi, A. Theoretical study of the electronic structure of mono-chloride of lanthanum molecule including spin-orbit coupling effect. J Mol Model 24, 100 (2018). https://doi.org/10.1007/s00894-018-3579-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3579-x